FXYD protein isoforms differentially modulate human Na/K pump function
- PMID: 33231612
- PMCID: PMC7690937
- DOI: 10.1085/jgp.202012660
FXYD protein isoforms differentially modulate human Na/K pump function
Abstract
Tight regulation of the Na/K pump is essential for cellular function because this heteromeric protein builds and maintains the electrochemical gradients for Na+ and K+ that energize electrical signaling and secondary active transport. We studied the regulation of the ubiquitous human α1β1 pump isoform by five human FXYD proteins normally located in muscle, kidney, and neurons. The function of Na/K pump α1β1 expressed in Xenopus oocytes with or without FXYD isoforms was evaluated using two-electrode voltage clamp and patch clamp. Through evaluation of the partial reactions in the absence of K+ but presence of Na+ in the external milieu, we demonstrate that each FXYD subunit alters the equilibrium between E1P(3Na) and E2P, the phosphorylated conformations with Na+ occluded and free from Na+, respectively, thereby altering the apparent affinity for Na+. This modification of Na+ interaction shapes the small effects of FXYD proteins on the apparent affinity for external K+ at physiological Na+. FXYD6 distinctively accelerated both the Na+-deocclusion and the pump-turnover rates. All FXYD isoforms altered the apparent affinity for intracellular Na+ in patches, an effect that was observed only in the presence of intracellular K+. Therefore, FXYD proteins alter the selectivity of the pump for intracellular ions, an effect that could be due to the altered equilibrium between E1 and E2, the two major pump conformations, and/or to small changes in ion affinities that are exacerbated when both ions are present. Lastly, we observed a drastic reduction of Na/K pump surface expression when it was coexpressed with FXYD1 or FXYD6, with the former being relieved by injection of PKA's catalytic subunit into the oocyte. Our results indicate that a prominent effect of FXYD1 and FXYD6, and plausibly other FXYDs, is the regulation of Na/K pump trafficking.
© 2020 Meyer et al.
Figures


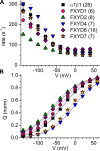
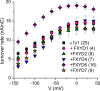
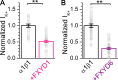
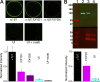

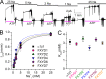

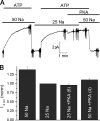
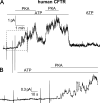
Comment in
-
What FXYDs fix.J Gen Physiol. 2021 Jun 7;153(6):e202012845. doi: 10.1085/jgp.202012845. J Gen Physiol. 2021. PMID: 33871566 Free PMC article.
Similar articles
-
FXYD proteins reverse inhibition of the Na+-K+ pump mediated by glutathionylation of its beta1 subunit.J Biol Chem. 2011 May 27;286(21):18562-72. doi: 10.1074/jbc.M110.184101. Epub 2011 Mar 30. J Biol Chem. 2011. PMID: 21454534 Free PMC article.
-
FXYD6 is a novel regulator of Na,K-ATPase expressed in the inner ear.J Biol Chem. 2007 Mar 9;282(10):7450-6. doi: 10.1074/jbc.M609872200. Epub 2007 Jan 5. J Biol Chem. 2007. PMID: 17209044
-
Phospholemman (FXYD1) raises the affinity of the human α1β1 isoform of Na,K-ATPase for Na ions.Biochemistry. 2011 May 10;50(18):3736-48. doi: 10.1021/bi2001714. Epub 2011 Apr 15. Biochemistry. 2011. PMID: 21449573
-
Function of FXYD proteins, regulators of Na, K-ATPase.J Bioenerg Biomembr. 2005 Dec;37(6):387-92. doi: 10.1007/s10863-005-9476-x. J Bioenerg Biomembr. 2005. PMID: 16691470 Review.
-
FXYD proteins: new tissue-specific regulators of the ubiquitous Na,K-ATPase.Sci STKE. 2003 Jan 21;2003(166):RE1. doi: 10.1126/stke.2003.166.re1. Sci STKE. 2003. PMID: 12538882 Review.
Cited by
-
Role of a conserved ion-binding site tyrosine in ion selectivity of the Na+/K+ pump.J Gen Physiol. 2022 Jul 4;154(7):e202113039. doi: 10.1085/jgp.202113039. Epub 2022 Jun 3. J Gen Physiol. 2022. PMID: 35657726 Free PMC article.
-
Mechanisms mediating effects of cardiotonic steroids in mammalian blood cells.Front Pharmacol. 2025 Mar 24;16:1520927. doi: 10.3389/fphar.2025.1520927. eCollection 2025. Front Pharmacol. 2025. PMID: 40196366 Free PMC article. Review.
-
Effect of Cations on ATP Binding to the N-domain of Na+, K+-ATPase.J Fluoresc. 2025 Jul;35(7):5521-5534. doi: 10.1007/s10895-024-03922-3. Epub 2024 Sep 19. J Fluoresc. 2025. PMID: 39298054
-
The Na+/K+ pump dominates control of glycolysis in hippocampal dentate granule cells.Elife. 2022 Oct 12;11:e81645. doi: 10.7554/eLife.81645. Elife. 2022. PMID: 36222651 Free PMC article.
-
Identification of Candidate Genes Associated with Yak Body Size Using a Genome-Wide Association Study and Multiple Populations of Information.Animals (Basel). 2023 Apr 26;13(9):1470. doi: 10.3390/ani13091470. Animals (Basel). 2023. PMID: 37174506 Free PMC article.
References
-
- Artigas P., Al’aref S.J., Hobart E.A., Díaz L.F., Sakaguchi M., Straw S., and Andersen O.S.. 2006. 2,3-butanedione monoxime affects cystic fibrosis transmembrane conductance regulator channel function through phosphorylation-dependent and phosphorylation-independent mechanisms: the role of bilayer material properties. Mol. Pharmacol. 70:2015–2026. 10.1124/mol.106.026070 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

