Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients
- PMID: 33231615
- PMCID: PMC7684031
- DOI: 10.1084/jem.20201707
Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients
Abstract
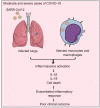
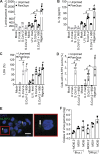
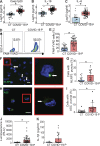
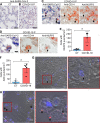
Severe cases of COVID-19 are characterized by a strong inflammatory process that may ultimately lead to organ failure and patient death. The NLRP3 inflammasome is a molecular platform that promotes inflammation via cleavage and activation of key inflammatory molecules including active caspase-1 (Casp1p20), IL-1β, and IL-18. Although participation of the inflammasome in COVID-19 has been highly speculated, the inflammasome activation and participation in the outcome of the disease are unknown. Here we demonstrate that the NLRP3 inflammasome is activated in response to SARS-CoV-2 infection and is active in COVID-19 patients. Studying moderate and severe COVID-19 patients, we found active NLRP3 inflammasome in PBMCs and tissues of postmortem patients upon autopsy. Inflammasome-derived products such as Casp1p20 and IL-18 in the sera correlated with the markers of COVID-19 severity, including IL-6 and LDH. Moreover, higher levels of IL-18 and Casp1p20 are associated with disease severity and poor clinical outcome. Our results suggest that inflammasomes participate in the pathophysiology of the disease, indicating that these platforms might be a marker of disease severity and a potential therapeutic target for COVID-19.
© 2020 Rodrigues et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures




References
-
- Barton, K. 2020. MuMIn: Multi-Model Inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn (accessed April 14, 2020)
-
- Bolker, B., and Robinson D.. 2020. broom.mixed: Tidying Methods for Mixed Models. R package version 0.2.6. https://CRAN.R-project.org/package=broom.mixed (accessed May 17, 2020)
-
- Brooks, M.E., Kristensen K., van Benthem K.J., Magnusson A., Berg C.W., Nielsen A., Skaug H.J., Maechler M., and Bolker B.M.. 2017. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 9:378–400. 10.32614/RJ-2017-066 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

