Limited rejuvenation of aged hematopoietic stem cells in young bone marrow niche
- PMID: 33231616
- PMCID: PMC7690939
- DOI: 10.1084/jem.20192283
Limited rejuvenation of aged hematopoietic stem cells in young bone marrow niche
Abstract
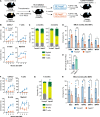
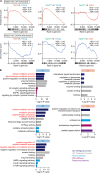
Hematopoietic stem cells (HSCs) exhibit functional alterations, such as reduced regenerative capacity and myeloid-biased differentiation, with age. The HSC niche, which is essential for the maintenance of HSCs, also undergoes marked changes with aging. However, it has been technically challenging to directly evaluate the contribution of niche aging to age-associated HSC alterations without niche-damaging myeloablation in HSC transplantation assays. We herein transplanted an excess of aged HSCs into young mice without preconditioning. Although aged HSCs successfully engrafted in the intact young bone marrow niche, they poorly regenerated downstream progenitors and exhibited persistent myeloid-biased differentiation, resulting in no significant functional rejuvenation. Transcriptome and methylome analyses revealed that the young niche largely restored the transcriptional profile of aged HSCs, but not their DNA methylation profiles. Therefore, the restoration of the young niche is insufficient for rejuvenating HSC functions, highlighting a key role for age-associated cell-intrinsic defects in HSC aging.
© 2020 Kuribayashi et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures







Similar articles
-
Mechanisms and rejuvenation strategies for aged hematopoietic stem cells.J Hematol Oncol. 2020 Apr 6;13(1):31. doi: 10.1186/s13045-020-00864-8. J Hematol Oncol. 2020. PMID: 32252797 Free PMC article. Review.
-
Harnessing matrix stiffness to engineer a bone marrow niche for hematopoietic stem cell rejuvenation.Cell Stem Cell. 2023 Apr 6;30(4):378-395.e8. doi: 10.1016/j.stem.2023.03.005. Cell Stem Cell. 2023. PMID: 37028404
-
Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche.Nat Med. 2018 Jun;24(6):782-791. doi: 10.1038/s41591-018-0030-x. Epub 2018 May 7. Nat Med. 2018. PMID: 29736022 Free PMC article.
-
Alteration of long- and short-term hematopoietic stem cell ratio causes myeloid-biased hematopoiesis.Elife. 2025 Aug 27;13:RP95880. doi: 10.7554/eLife.95880. Elife. 2025. PMID: 40864176 Free PMC article.
-
Hand in hand: intrinsic and extrinsic drivers of aging and clonal hematopoiesis.Exp Hematol. 2020 Nov;91:1-9. doi: 10.1016/j.exphem.2020.09.197. Epub 2020 Sep 28. Exp Hematol. 2020. PMID: 32991978 Free PMC article. Review.
Cited by
-
Decreased non-neurogenic acetylcholine in bone marrow triggers age-related defective stem/progenitor cell homing.Nat Commun. 2025 Jul 1;16(1):5475. doi: 10.1038/s41467-025-60515-9. Nat Commun. 2025. PMID: 40593589 Free PMC article.
-
Aging of the Hematopoietic Stem Cell Niche: New Tools to Answer an Old Question.Front Immunol. 2021 Nov 11;12:738204. doi: 10.3389/fimmu.2021.738204. eCollection 2021. Front Immunol. 2021. PMID: 34858399 Free PMC article. Review.
-
An iron rheostat controls hematopoietic stem cell fate.Cell Stem Cell. 2024 Mar 7;31(3):378-397.e12. doi: 10.1016/j.stem.2024.01.011. Epub 2024 Feb 22. Cell Stem Cell. 2024. PMID: 38402617 Free PMC article.
-
Aging and Clonal Behavior of Hematopoietic Stem Cells.Int J Mol Sci. 2022 Feb 9;23(4):1948. doi: 10.3390/ijms23041948. Int J Mol Sci. 2022. PMID: 35216063 Free PMC article. Review.
-
Hematopoietic Stem Cells and the Immune System in Development and Aging.Int J Mol Sci. 2023 Mar 20;24(6):5862. doi: 10.3390/ijms24065862. Int J Mol Sci. 2023. PMID: 36982935 Free PMC article. Review.
References
-
- Beerman, I., Bhattacharya D., Zandi S., Sigvardsson M., Weissman I.L., Bryder D., and Rossi D.J.. 2010. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. USA. 107:5465–5470. 10.1073/pnas.1000834107 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

