Estimated SARS-CoV-2 Seroprevalence in the US as of September 2020
- PMID: 33231628
- PMCID: PMC7686880
- DOI: 10.1001/jamainternmed.2020.7976
Estimated SARS-CoV-2 Seroprevalence in the US as of September 2020
Abstract
Importance: Case-based surveillance of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection likely underestimates the true prevalence of infections. Large-scale seroprevalence surveys can better estimate infection across many geographic regions.
Objective: To estimate the prevalence of persons with SARS-CoV-2 antibodies using residual sera from commercial laboratories across the US and assess changes over time.
Design, setting, and participants: This repeated, cross-sectional study conducted across all 50 states, the District of Columbia, and Puerto Rico used a convenience sample of residual serum specimens provided by persons of all ages that were originally submitted for routine screening or clinical management from 2 private clinical commercial laboratories. Samples were obtained during 4 collection periods: July 27 to August 13, August 10 to August 27, August 24 to September 10, and September 7 to September 24, 2020.
Exposures: Infection with SARS-CoV-2.
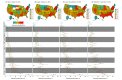
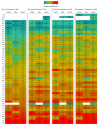
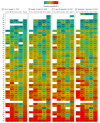
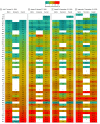
Main outcomes and measures: The proportion of persons previously infected with SARS-CoV-2 as measured by the presence of antibodies to SARS-CoV-2 by 1 of 3 chemiluminescent immunoassays. Iterative poststratification was used to adjust seroprevalence estimates to the demographic profile and urbanicity of each jurisdiction. Seroprevalence was estimated by jurisdiction, sex, age group (0-17, 18-49, 50-64, and ≥65 years), and metropolitan/nonmetropolitan status.
Results: Of 177 919 serum samples tested, 103 771 (58.3%) were from women, 26 716 (15.0%) from persons 17 years or younger, 47 513 (26.7%) from persons 65 years or older, and 26 290 (14.8%) from individuals living in nonmetropolitan areas. Jurisdiction-level seroprevalence over 4 collection periods ranged from less than 1% to 23%. In 42 of 49 jurisdictions with sufficient samples to estimate seroprevalence across all periods, fewer than 10% of people had detectable SARS-CoV-2 antibodies. Seroprevalence estimates varied between sexes, across age groups, and between metropolitan/nonmetropolitan areas. Changes from period 1 to 4 were less than 7 percentage points in all jurisdictions and varied across sites.
Conclusions and relevance: This cross-sectional study found that as of September 2020, most persons in the US did not have serologic evidence of previous SARS-CoV-2 infection, although prevalence varied widely by jurisdiction. Biweekly nationwide testing of commercial clinical laboratory sera can play an important role in helping track the spread of SARS-CoV-2 in the US.
Conflict of interest statement
Figures
Comment in
-
Antibodies, Immunity, and COVID-19.JAMA Intern Med. 2021 Apr 1;181(4):460-462. doi: 10.1001/jamainternmed.2020.7986. JAMA Intern Med. 2021. PMID: 33231673 Free PMC article. No abstract available.
-
SARS-CoV-2 Seroprevalence Data to Guide Local Public Health Interventions.JAMA Intern Med. 2021 Jul 1;181(7):1014. doi: 10.1001/jamainternmed.2021.0074. JAMA Intern Med. 2021. PMID: 33720309 No abstract available.
References
-
- California Department of Public Health . CDC confirms possible first instance of COVID-19 community transmission in California. Accessed June 17, 2020. https://www.cdph.ca.gov/Programs/OPA/Pages/NR20-006.aspx
-
- Washington State Department of Health . Additional cases of COVID-19 in Washington state. Accessed June 17, 2020. https://www.doh.wa.gov/Newsroom/Articles/ID/1103/Additional-Cases-of-COV...
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

