Effect of Nebulized Magnesium vs Placebo Added to Albuterol on Hospitalization Among Children With Refractory Acute Asthma Treated in the Emergency Department: A Randomized Clinical Trial
- PMID: 33231663
- PMCID: PMC7686869
- DOI: 10.1001/jama.2020.19839
Effect of Nebulized Magnesium vs Placebo Added to Albuterol on Hospitalization Among Children With Refractory Acute Asthma Treated in the Emergency Department: A Randomized Clinical Trial
Abstract
Importance: While intravenous magnesium decreases hospitalizations in refractory pediatric acute asthma, it is variably used because of invasiveness and safety concerns. The benefit of nebulized magnesium to prevent hospitalization is unknown.
Objective: To evaluate the effectiveness of nebulized magnesium in children with acute asthma remaining in moderate or severe respiratory distress after initial therapy.
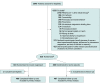
Design, setting, and participants: A randomized double-blind parallel-group clinical trial from September 26, 2011, to November 19, 2019, in 7 tertiary-care pediatric emergency departments in Canada. The participants were otherwise healthy children aged 2 to 17 years with moderate to severe asthma defined by a Pediatric Respiratory Assessment Measure (PRAM) score of 5 or greater (on a 12-point scale) after a 1-hour treatment with an oral corticosteroid and 3 inhaled albuterol and ipratropium treatments. Of 5846 screened patients, 4332 were excluded for criteria, 273 declined participation, 423 otherwise excluded, 818 randomized, and 816 analyzed.
Interventions: Participants were randomized to 3 nebulized albuterol treatments with either magnesium sulfate (n = 410) or 5.5% saline placebo (n = 408).
Main outcomes and measures: The primary outcome was hospitalization for asthma within 24 hours. Secondary outcomes included PRAM score; respiratory rate; oxygen saturation at 60, 120, 180, and 240 minutes; blood pressure at 20, 40, 60, 120, 180, and 240 minutes; and albuterol treatments within 240 minutes.
Results: Among 818 randomized patients (median age, 5 years; 63% males), 816 completed the trial (409 received magnesium; 407, placebo). A total of 178 of the 409 children who received magnesium (43.5%) were hospitalized vs 194 of the 407 who received placebo (47.7%) (difference, -4.2%; absolute risk difference 95% [exact] CI, -11% to 2.8%]; P = .26). There were no significant between-group differences in changes from baseline to 240 minutes in PRAM score (difference of changes, 0.14 points [95% CI, -0.23 to 0.50]; P = .46); respiratory rate (0.17 breaths/min [95% CI, -1.32 to 1.67]; P = .82); oxygen saturation (-0.04% [95% CI, -0.53% to 0.46%]; P = .88); systolic blood pressure (0.78 mm Hg [95% CI, -1.48 to 3.03]; P = .50); or mean number of additional albuterol treatments (magnesium: 1.49, placebo: 1.59; risk ratio, 0.94 [95% CI, 0.79 to 1.11]; P = .47). Nausea/vomiting or sore throat/nose occurred in 17 of the 409 children who received magnesium (4%) and 5 of the 407 who received placebo (1%).
Conclusions and relevance: Among children with refractory acute asthma in the emergency department, nebulized magnesium with albuterol, compared with placebo with albuterol, did not significantly decrease the hospitalization rate for asthma within 24 hours. The findings do not support use of nebulized magnesium with albuterol among children with refractory acute asthma.
Trial registration: ClinicalTrials.gov Identifier: NCT01429415.
Conflict of interest statement
Figures

References
-
- Moorman JE, Rudd RA, Johnson CA, et al. ; Centers for Disease Control and Prevention (CDC) . National surveillance for asthma: United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54. - PubMed
-
- Global Initiative for Asthma Global Initiative for Asthma (GINA). Accessed April 5, 2020. http://www.ginasthma.org

