Random urine drug testing among patients receiving opioid therapy for cancer pain
- PMID: 33231885
- PMCID: PMC10015495
- DOI: 10.1002/cncr.33326
Random urine drug testing among patients receiving opioid therapy for cancer pain
Abstract
Background: There is limited information regarding the true frequency of nonmedical opioid use (NMOU) among patients receiving opioid therapy for cancer pain. Data to guide patient selection for urine drug testing (UDT) as well as the timing and frequency of ordering UDT are insufficient. This study examined the frequency of abnormal UDT among patients with cancer who underwent random UDT and their characteristics.
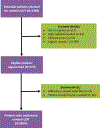
Methods: Demographic and clinical information for patients with cancer who underwent random UDT were retrospectively reviewed and compared with a historical cohort that underwent targeted UDT. Random UDT was ordered regardless of a patient's risk potential for NMOU. Targeted UDT was ordered on the basis of a physician's estimation of a patient's risk for NMOU.
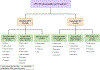
Results: In all, 552 of 573 eligible patients (96%) underwent random UDT. Among these patients, 130 (24%) had 1 or more abnormal results; 38 of the 88 patients (43%) who underwent targeted UDT had 1 or more abnormal results. When marijuana was excluded, 15% of the random group and 37% of the targeted group had abnormal UDT findings (P < .001). It took a shorter time from the initial consultation to detect 1 or more abnormalities with the random test than the targeted test (median, 130 vs 274 days; P = .02). Abnormal random UDT was independently associated with younger age (P < .0001), male sex (P = .03), Cut Down, Annoyed, Guilty, and Eye Opener-Adapted to Include Drugs positivity (P = .001), and higher Edmonton Symptom Assessment System anxiety (P = .01).
Conclusions: Approximately 1 in 4 patients receiving opioids for cancer pain at a supportive care clinic who underwent random UDT had 1 or more abnormalities. Random UDT detected abnormalities earlier than the targeted test. These findings suggest that random UDT is justified among patients with cancer pain.
Keywords: cancer pain; opioid; random; targeted; urine drug test.
© 2020 American Cancer Society.
Figures
References
-
- Stjernsward J WHO cancer pain relief programme. Cancer Surv. 1988;7:195–208. - PubMed
-
- Stjernsward J, Colleau SM, Ventafridda V. The World Health Organization Cancer Pain and Palliative Care Program. Past, present, and future. J Pain Symptom Manage. 1996;12:65–72. - PubMed
-
- Passik SD, Portenoy RK, Ricketts PL. Substance abuse issues in cancer patients. Part 1: prevalence and diagnosis. Oncology (Williston Park). 1998;12:517–521. - PubMed
-
- Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18:S76–S82. - PubMed
-
- Chou R 2009 clinical guidelines from the American Pain Society and the American Academy of Pain Medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119:469–477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

