Assessment of COVID-19 Treatment containing both Hydroxychloroquine and Azithromycin: A natural clinical trial
- PMID: 33231925
- PMCID: PMC7744890
- DOI: 10.1111/ijcp.13856
Assessment of COVID-19 Treatment containing both Hydroxychloroquine and Azithromycin: A natural clinical trial
Abstract
The goal of this study was to assess the clinical effectiveness and safety profile of the COVID-19 treatment protocol (containing both hydroxychloroquine (HCQ) and azithromycin) in an Iraqi specialised hospital.
Methods: This prospective study used a pre- and post-intervention design without a comparison group. The intervention was routine Ministry of Health (MOH) approved the management of COVID-19 for all patients. The study was conducted in a public healthcare setting in Baghdad, Iraq from March 1st to May 25, 2020. The study outcome measures included the changes in clinical and biochemical parameters during the hospitalisation period. Paired t-test and Chi-square test were used to compare the measures of vital signs, lab tests and symptoms before and after treatment.
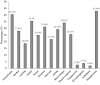
Results: The study included 161 patients who were admitted with positive RT-PCR and clinical symptoms of COVID-19. In terms of severity, 53 (32.9%) patients had amild condition, 47 (29.2%) had moderate condition, 35 (21.7%) had severe condition and 26 (16.1%) had critical condition. Most patients (84.5%) recovered and were discharged without symptoms after testing negative with RT-PCR, while 11 (6.8%) patients died during the study period. The signs and symptoms of COVID-19 were reduced significantly in response to a therapy regimen containing HCQ and azithromycin. The most common reported side effects were stomach pain, hypoglycemia, dizziness, itching, skin rash, QT prolongation, arrhythmia, and conjunctivitis.
Conclusions: This natural trial showed that the COVID-19 regimen containing both HCQ and azithromycin can be helpful to promote the recovery of most patients and reduced their signs and symptoms significantly. It also shows some manageable side effects mostly those related to heart rhythm. In the absence of FDA-approved medications to treat COVID-19, the repurposing of HCQ and azithromycin to control the disease signs and symptoms can be useful.
Keywords: COVID-19; azithromycin; effectiveness; hydroxychloroquine; natural trial; safety; treatment.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
No conflict of interest to declare and the study received no fund.
Figures


References
-
- Tamblyn S. Clinical management of patients with moderate to severe COVID‐19 ‐ Interim Guidance. Public Heal Agency Canada. 2020;1‐20.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
