Pharmacokinetics of solifenacin in pediatric populations with overactive bladder or neurogenic detrusor overactivity
- PMID: 33231929
- PMCID: PMC7685239
- DOI: 10.1002/prp2.684
Pharmacokinetics of solifenacin in pediatric populations with overactive bladder or neurogenic detrusor overactivity
Abstract
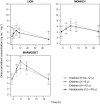
The aim of this investigation was to characterize and compare the pharmacokinetics (PK) of the antimuscarinic drug solifenacin in pediatric patients with overactive bladder (OAB) or neurogenic detrusor overactivity (NDO) utilizing data from three phase III trials. LION was a placebo-controlled, 12-week trial in children (5-<12 years) and adolescents (12-<18 years) with OAB. MONKEY and MARMOSET were open-label, 52-week trials in children and adolescents or younger children (6 months-<5 years), respectively, with NDO. During the trials, solifenacin doses could be titrated to weight-adjusted pediatric equivalent doses (PEDs) of 2.5, 5, 7.5, or 10 mg day-1 . Nonlinear mixed effects modeling was used to develop population PK models to characterize the PK in patients with either OAB or NDO. Overall, 194 children and adolescents received solifenacin. At the time of PK sampling, the majority (119/164 [72.6%] patients) were receiving PED10 once daily. All population models included first-order oral absorption, a lag time, and interindividual variability. PK analysis showed that apparent clearance was similar in both patient populations. Mean apparent oral plasma clearance (CL/F), apparent volume of distribution during the terminal phase (Vz /F), and terminal half-life (t1/2 ) were higher in adolescents than in children, but median time to maximum plasma concentration (tmax ) was similar. Dose-normalized exposure results were similar for both younger and older patients with OAB or NDO. In conclusion, population PK modeling was used to successfully characterize solifenacin PK in pediatric patients with OAB or NDO. Similar solifenacin PK characteristics were observed in both populations.
Trial registration: ClinicalTrials.gov NCT01565707 NCT01565694 NCT01981954.
Keywords: nephrology - urology; pediatrics - children; pharmacokinetics.
© 2020 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
All the authors are current or former employees of either Astellas Pharma Europe B.V. or Astellas Pharma Global Development Inc. The authors thank the LION, MONKEY, and MARMOSET trial investigators, and all the patients and their parents/legal representatives who took part in the trials. These trials were funded by Astellas Pharma Europe B.V. Medical writing support was provided by Michael Parsons, PhD, CMPP of Elevate Scientific Solutions, who provided permission to be named in this section, and funded by Astellas Pharma Global Development, Inc. The authors also thank the contract research organization involved in conducting these trials, PPD Global Limited, Cambridge, UK.
Figures

References
-
- Austin PF, Bauer SB, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the standardization committee of the International Children's Continence Society. Neurourol Urodyn. 2016;35:471‐481. - PubMed
-
- Drake MJ. Do we need a new definition of the overactive bladder syndrome? ICI‐RS 2013. Neurourol Urodyn. 2014;33:622‐624. - PubMed
-
- Chung JM, Lee SD, Kang DI, et al. Prevalence and associated factors of overactive bladder in Korean children 5–13 years old: a nationwide multicenter study. Urology. 2009;73:63‐67. - PubMed
-
- Hellström A, Hanson E, Hansson S, Hjälmås K, Jodal U. Micturition habits and incontinence at age 17 – reinvestigation of a cohort studied at age 7. Br J Urol. 1995;76:231‐234. - PubMed
-
- Kajiwara M, Inoue K, Mutaguchi K, Usui T. The prevalence of overactive bladder and nocturnal enuresis in Japanese early adolescents : a questionnaire survey. Hinyokika Kiyo. 2006;52:107‐111. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

