Understanding how temperature shifts could impact infectious disease
- PMID: 33232316
- PMCID: PMC7685459
- DOI: 10.1371/journal.pbio.3000938
Understanding how temperature shifts could impact infectious disease
Abstract
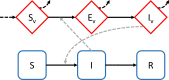
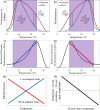
Climate change is expected to have complex effects on infectious diseases, causing some to increase, others to decrease, and many to shift their distributions. There have been several important advances in understanding the role of climate and climate change on wildlife and human infectious disease dynamics over the past several years. This essay examines 3 major areas of advancement, which include improvements to mechanistic disease models, investigations into the importance of climate variability to disease dynamics, and understanding the consequences of thermal mismatches between host and parasites. Applying the new information derived from these advances to climate-disease models and addressing the pressing knowledge gaps that we identify should improve the capacity to predict how climate change will affect disease risk for both wildlife and humans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

