The prospective Hemophilia Inhibitor PUP Study reveals distinct antibody signatures prior to FVIII inhibitor development
- PMID: 33232473
- PMCID: PMC7686884
- DOI: 10.1182/bloodadvances.2020002731
The prospective Hemophilia Inhibitor PUP Study reveals distinct antibody signatures prior to FVIII inhibitor development
Abstract
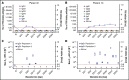
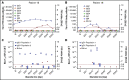
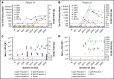
Preventing factor VIII (FVIII) inhibitors following replacement therapies with FVIII products in patients with hemophilia A remains an unmet medical need. Better understanding of the early events of evolving FVIII inhibitors is essential for risk identification and the design of novel strategies to prevent inhibitor development. The Hemophilia Inhibitor Previously Untreated Patients (PUPs) Study (HIPS; www.clinicaltrials.gov #NCT01652027) is the first prospective cohort study to evaluate comprehensive changes in the immune system during the first 50 exposure days (EDs) to FVIII in patients with severe hemophilia A. HIPS participants were enrolled prior to their first exposure to FVIII or blood products ("true PUPs") and were evaluated for different immunological and clinical parameters at specified time points during their first 50 EDs to a single source of recombinant FVIII. Longitudinal antibody data resulting from this study indicate that there are 4 subgroups of patients expressing distinct signatures of FVIII-binding antibodies. Subgroup 1 did not develop any detectable FVIII-binding immunoglobulin G (IgG) antibodies. Subgroup 2 developed nonneutralizing, FVIII-binding IgG1 antibodies, but other FVIII-binding IgG subclasses were not observed. Subgroup 3 developed transient FVIII inhibitors associated with FVIII-binding IgG1 antibodies, similar to subgroup 2. Subgroup 4 developed persistent FVIII inhibitors associated with an initial development of high-affinity, FVIII-binding IgG1 antibodies, followed by IgG3 and IgG4 antibodies. Appearance of FVIII-binding IgG3 was always associated with persistent FVIII inhibitors and the subsequent development of FVIII-binding IgG4. Some of the antibody signatures identified in HIPS could serve as candidates for early biomarkers of FVIII inhibitor development.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: B.M.R. was an employee of Baxalta Innovations at the time of the study but left after study completion. B.G. is an employee of Baxalta Innovations and holds Takeda stock options. C.J.H. was an employee of Baxalta Innovations at the time of the study but is now an employee of Takeda Pharmaceutical (Cambridge, MA) and holds Takeda stock options. V.B. and H.S. received institutional research funding from Baxalta Innovations. J. Bowen received research funding from the study sponsor, University of Texas Health Science Center (Houston, TX). J. Blatny has received consultation/speakers fees from Takeda, Novo Nordisk, Sobi, LFB, Roche, Pfizer, CSL Behring, and Octapharma. K.F. has received unrestricted institutional research grants from CSL Behring and Novo Nordisk and institutional consultancy fees from Grifols, Takeda, Novo Nordisk, and Roche. E.S.M. has received advisory board fees from Bayer and Takeda
Figures




References
-
- Lusher JM, Arkin S, Abildgaard CF, Schwartz RS; Kogenate Previously Untreated Patient Study Group . Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and development of inhibitors. N Engl J Med. 1993;328(7):453-459. - PubMed
-
- Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9(4):418-435. - PubMed
-
- Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med. 2016;374(21):2054-2064. - PubMed
-
- Morfini M, Haya S, Tagariello G, et al. European study on orthopaedic status of haemophilia patients with inhibitors. Haemophilia. 2007;13(5):606-612. - PubMed

