Immunohistochemical Detection of Synuclein Pathology in Skin in Idiopathic Rapid Eye Movement Sleep Behavior Disorder and Parkinsonism
- PMID: 33232556
- PMCID: PMC10123546
- DOI: 10.1002/mds.28399
Immunohistochemical Detection of Synuclein Pathology in Skin in Idiopathic Rapid Eye Movement Sleep Behavior Disorder and Parkinsonism
Abstract
Background: Recent studies reported abnormal alpha-synuclein deposition in biopsy-accessible sites of the peripheral nervous system in Parkinson's disease (PD). This has considerable implications for clinical diagnosis. Moreover, if deposition occurs early, it may enable tissue diagnosis of prodromal PD.
Objective: The aim of this study was to develop and test an automated bright-field immunohistochemical assay of cutaneous pathological alpha-synuclein deposition in patients with idiopathic rapid eye movement sleep behavior disorder, PD, and atypical parkinsonism and in control subjects.
Methods: For assay development, postmortem skin biopsies were taken from 28 patients with autopsy-confirmed Lewy body disease and 23 control subjects. Biopsies were stained for pathological alpha-synuclein in automated stainers using a novel dual-immunohistochemical assay for serine 129-phosphorylated alpha-synuclein and pan-neuronal marker protein gene product 9.5. After validation, single 3-mm punch skin biopsies were taken from the cervical 8 paravertebral area from 79 subjects (28 idiopathic rapid eye movement sleep behavior disorder, 20 PD, 10 atypical parkinsonism, and 21 control subjects). Raters blinded to clinical diagnosis assessed the biopsies.
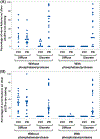
Results: The immunohistochemistry assay differentiated alpha-synuclein pathology from nonpathological-appearing alpha-synuclein using combined phosphatase and protease treatments. Among autopsy samples, 26 of 28 Lewy body samples and none of the 23 controls were positive. Among living subjects, punch biopsies were positive in 23 (82%) subjects with idiopathic rapid eye movement sleep behavior disorder, 14 (70%) subjects with PD, 2 (20%) subjects with atypical parkinsonism, and none (0%) of the control subjects. After a 3-year follow-up, eight idiopathic rapid eye movement sleep behavior disorder subjects phenoconverted to defined neurodegenerative syndromes, in accordance with baseline biopsy results.
Conclusion: Even with a single 3-mm punch biopsy, there is considerable promise for using pathological alpha-synuclein deposition in skin to diagnose both clinical and prodromal PD. © 2020 International Parkinson and Movement Disorder Society.
Keywords: PGP9.5; Parkinson's disease; RBD; UCHL1; alpha-synuclein; skin biopsy.
© 2020 International Parkinson and Movement Disorder Society.
Conflict of interest statement
Figures



References
-
- Donadio V, Incensi A, Leta V, et al. Skin nerve alpha-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology 2014;82(15):1362–1369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

