A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia
- PMID: 33232588
- PMCID: PMC7722692
- DOI: 10.1056/NEJMoa2031304
A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia
Abstract
Background: Convalescent plasma is frequently administered to patients with Covid-19 and has been reported, largely on the basis of observational data, to improve clinical outcomes. Minimal data are available from adequately powered randomized, controlled trials.
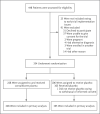
Methods: We randomly assigned hospitalized adult patients with severe Covid-19 pneumonia in a 2:1 ratio to receive convalescent plasma or placebo. The primary outcome was the patient's clinical status 30 days after the intervention, as measured on a six-point ordinal scale ranging from total recovery to death.
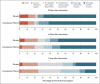
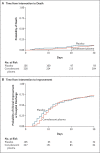
Results: A total of 228 patients were assigned to receive convalescent plasma and 105 to receive placebo. The median time from the onset of symptoms to enrollment in the trial was 8 days (interquartile range, 5 to 10), and hypoxemia was the most frequent severity criterion for enrollment. The infused convalescent plasma had a median titer of 1:3200 of total SARS-CoV-2 antibodies (interquartile range, 1:800 to 1:3200). No patients were lost to follow-up. At day 30 day, no significant difference was noted between the convalescent plasma group and the placebo group in the distribution of clinical outcomes according to the ordinal scale (odds ratio, 0.83; 95% confidence interval [CI], 0.52 to 1.35; P = 0.46). Overall mortality was 10.96% in the convalescent plasma group and 11.43% in the placebo group, for a risk difference of -0.46 percentage points (95% CI, -7.8 to 6.8). Total SARS-CoV-2 antibody titers tended to be higher in the convalescent plasma group at day 2 after the intervention. Adverse events and serious adverse events were similar in the two groups.
Conclusions: No significant differences were observed in clinical status or overall mortality between patients treated with convalescent plasma and those who received placebo. (PlasmAr ClinicalTrials.gov number, NCT04383535.).
Copyright © 2020 Massachusetts Medical Society.
Figures
References
-
- Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 — preliminary report: reply. N Engl J Med 2020;383:994-994. - PubMed
-
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006;145:599-609. - PubMed
-
- Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet 1979;2:1216-1217. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
