Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival
- PMID: 33232761
- PMCID: PMC9348585
- DOI: 10.1016/j.annonc.2020.11.007
Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival
Abstract
Background: Pathologic complete response (pCR) to neoadjuvant chemotherapy (NAC) is strongly associated with favorable outcome. We examined the utility of serial circulating tumor DNA (ctDNA) testing for predicting pCR and risk of metastatic recurrence.
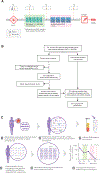
Patients and methods: Cell-free DNA (cfDNA) was isolated from 291 plasma samples of 84 high-risk early breast cancer patients treated in the neoadjuvant I-SPY 2 TRIAL with standard NAC alone or combined with MK-2206 (AKT inhibitor) treatment. Blood was collected at pretreatment (T0), 3 weeks after initiation of paclitaxel (T1), between paclitaxel and anthracycline regimens (T2), or prior to surgery (T3). A personalized ctDNA test was designed to detect up to 16 patient-specific mutations (from whole-exome sequencing of pretreatment tumor) in cfDNA by ultra-deep sequencing. The median follow-up time for survival analysis was 4.8 years.
Results: At T0, 61 of 84 (73%) patients were ctDNA positive, which decreased over time (T1: 35%; T2: 14%; and T3: 9%). Patients who remained ctDNA positive at T1 were significantly more likely to have residual disease after NAC (83% non-pCR) compared with those who cleared ctDNA (52% non-pCR; odds ratio 4.33, P = 0.012). After NAC, all patients who achieved pCR were ctDNA negative (n = 17, 100%). For those who did not achieve pCR (n = 43), ctDNA-positive patients (14%) had a significantly increased risk of metastatic recurrence [hazard ratio (HR) 10.4; 95% confidence interval (CI) 2.3-46.6]; interestingly, patients who did not achieve pCR but were ctDNA negative (86%) had excellent outcome, similar to those who achieved pCR (HR 1.4; 95% CI 0.15-13.5).
Conclusions: Lack of ctDNA clearance was a significant predictor of poor response and metastatic recurrence, while clearance was associated with improved survival even in patients who did not achieve pCR. Personalized monitoring of ctDNA during NAC of high-risk early breast cancer may aid in real-time assessment of treatment response and help fine-tune pCR as a surrogate endpoint of survival.
Keywords: breast cancer; circulating tumor DNA; neoadjuvant chemotherapy; pathologic complete response.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure The following authors are employees of Natera, Inc. (HS, H-TW, RS, AT, SS, HP, PB, AA, ML, BZ). LJVV is co-founder, stockholder, and part-time employee of Agendia NV. The remaining authors have declared no conflicts of interest.
Figures




References
-
- Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nature reviews Clinical oncology 2017; 14: 531–548. - PubMed
-
- Donaldson J, Park BH. Circulating Tumor DNA: Measurement and Clinical Utility. Annu Rev Med 2018; 69: 223–234. - PubMed
-
- Rossi G, Ignatiadis M. Promises and Pitfalls of Using Liquid Biopsy for Precision Medicine. Cancer Res 2019; 79: 2798–2804. - PubMed
-
- Garcia-Murillas I, Schiavon G, Weigelt B et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 2015; 7: 302ra133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

