SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells
- PMID: 33232769
- PMCID: PMC7680014
- DOI: 10.1016/j.vph.2020.106823
SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells
Abstract
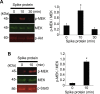
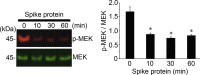
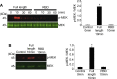
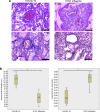
Currently, the world is suffering from the pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that uses angiotensin-converting enzyme 2 (ACE2) as a receptor to enter the host cells. So far, 60 million people have been infected with SARS-CoV-2, and 1.4 million people have died because of COVID-19 worldwide, causing serious health, economical, and sociological problems. However, the mechanism of the effect of SARS-CoV-2 on human host cells has not been defined. The present study reports that the SARS-CoV-2 spike protein alone without the rest of the viral components is sufficient to elicit cell signaling in lung vascular cells. The treatment of human pulmonary artery smooth muscle cells or human pulmonary artery endothelial cells with recombinant SARS-CoV-2 spike protein S1 subunit (Val16 - Gln690) at 10 ng/ml (0.13 nM) caused an activation of MEK phosphorylation. The activation kinetics was transient with a peak at 10 min. The recombinant protein that contains only the ACE2 receptor-binding domain of the SARS-CoV-2 spike protein S1 subunit (Arg319 - Phe541), on the other hand, did not cause this activation. Consistent with the activation of cell growth signaling in lung vascular cells by the SARS-CoV-2 spike protein, pulmonary vascular walls were found to be thickened in COVID-19 patients. Thus, SARS-CoV-2 spike protein-mediated cell growth signaling may participate in adverse cardiovascular/pulmonary outcomes, and this mechanism may provide new therapeutic targets to combat COVID-19.
Keywords: COVID-19; Cell signaling; Coronavirus; SARS-CoV-2; Vascular.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
None.
Figures
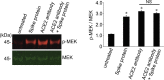
Update of
-
SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells.bioRxiv [Preprint]. 2020 Oct 12:2020.10.12.335083. doi: 10.1101/2020.10.12.335083. bioRxiv. 2020. Update in: Vascul Pharmacol. 2021 Apr;137:106823. doi: 10.1016/j.vph.2020.106823. PMID: 33052333 Free PMC article. Updated. Preprint.
References
-
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

