SARS-CoV-2 attachment to host cells is possibly mediated via RGD-integrin interaction in a calcium-dependent manner and suggests pulmonary EDTA chelation therapy as a novel treatment for COVID 19
- PMID: 33232865
- PMCID: PMC7642744
- DOI: 10.1016/j.imbio.2020.152021
SARS-CoV-2 attachment to host cells is possibly mediated via RGD-integrin interaction in a calcium-dependent manner and suggests pulmonary EDTA chelation therapy as a novel treatment for COVID 19
Abstract
SARS-CoV-2 is a highly contagious virus that has caused serious health crisis world-wide resulting into a pandemic situation. As per the literature, the SARS-CoV-2 is known to exploit humanACE2 receptors (similar toprevious SARS-CoV-1) for gaining entry into the host cell for invasion, infection, multiplication and pathogenesis. However, considering the higher infectivity of SARS-CoV-2 along with the complex etiology and pathophysiological outcomes seen in COVID-19 patients, it seems that there may be an alternate receptor for SARS-CoV-2. I performed comparative protein sequence analysis, database based gene expression profiling, bioinformatics based molecular docking using authentic tools and techniques for unveiling the molecular basis of high infectivity of SARS-CoV-2 as compared to previous known coronaviruses. My study revealed that SARS-CoV-2 (previously known as 2019-nCoV) harbors a RGD motif in its receptor binding domain (RBD) and the motif is absent in all other previously known SARS-CoVs. The RGD motif is well known for its role in cell-attachment and cell-adhesion. My hypothesis is that the SARS-CoV-2 may be (via RGD) exploiting integrins, that have high expression in lungs and all other vital organs, for invading host cells. However, an experimental verification is required. The expression of ACE2, which is a known receptor for SARS-CoV-2, was found to be negligible in lungs. I assume that higher infectivity of SARS-CoV-2 could be due to this RGD-integrin mediated acquired cell-adhesive property. Gene expression profiling revealed that expression of integrins is significantly high in lung cells, in particular αvβ6, α5β1, αvβ8 and an ECM protein, ICAM1. The molecular docking experiment showed the RBD of spike protein binds with integrins precisely at RGD motif in a similar manner as a synthetic RGD peptide binds to integrins as found by other researchers. SARS-CoV-2 spike protein has a number of phosphorylation sites that can induce cAMP, PKC, Tyr signaling pathways. These pathways either activate calcium ion channels or get activated by calcium. In fact, integrins have calcium & metal binding sites that were predicted around and in vicinity of RGD-integrin docking site in our analysis which suggests that RGD-integrins interaction possibly occurs in calcium-dependent manner. The higher expression of integrins in lungs along with their previously known high binding affinity (~KD = 4.0 nM) for virus RGD motif could serve as a possible explanation for high infectivity of SARS-CoV-2. On the contrary, human ACE2 has lower expression in lungs and its high binding affinity (~KD = 15 nM) for spike RBD alone could not manifest significant virus-host attachment. This suggests that besides human ACE2, an additional or alternate receptor for SARS-CoV-2 is likely to exist. A highly relevant evidence never reported earlier which corroborate in favor of RGD-integrins mediated virus-host attachment is an unleashed cytokine storm which causes due to activation of TNF-α and IL-6 activation; and integrins role in their activation is also well established. Altogether, the current study has highlighted possible role of calcium and other divalent ions in RGD-integrins interaction for virus invasion into host cells and suggested that lowering divalent ion in lungs could avert virus-host cells attachment.
Copyright © 2020 Elsevier GmbH. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

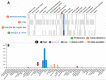

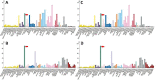

Similar articles
-
Biological and Clinical Consequences of Integrin Binding via a Rogue RGD Motif in the SARS CoV-2 Spike Protein.Viruses. 2021 Jan 20;13(2):146. doi: 10.3390/v13020146. Viruses. 2021. PMID: 33498225 Free PMC article. Review.
-
V367F Mutation in SARS-CoV-2 Spike RBD Emerging during the Early Transmission Phase Enhances Viral Infectivity through Increased Human ACE2 Receptor Binding Affinity.J Virol. 2021 Jul 26;95(16):e0061721. doi: 10.1128/JVI.00617-21. Epub 2021 Jul 26. J Virol. 2021. PMID: 34105996 Free PMC article.
-
Can the SARS-CoV-2 Spike Protein Bind Integrins Independent of the RGD Sequence?Front Cell Infect Microbiol. 2021 Nov 18;11:765300. doi: 10.3389/fcimb.2021.765300. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34869067 Free PMC article.
-
Withanone from Withania somnifera Attenuates SARS-CoV-2 RBD and Host ACE2 Interactions to Rescue Spike Protein Induced Pathologies in Humanized Zebrafish Model.Drug Des Devel Ther. 2021 Mar 11;15:1111-1133. doi: 10.2147/DDDT.S292805. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33737804 Free PMC article.
-
Inhibition of S-protein RBD and hACE2 Interaction for Control of SARSCoV- 2 Infection (COVID-19).Mini Rev Med Chem. 2021;21(6):689-703. doi: 10.2174/1389557520666201117111259. Mini Rev Med Chem. 2021. PMID: 33208074 Review.
Cited by
-
Antigenic sites in SARS-CoV-2 spike RBD show molecular similarity with pathogenic antigenic determinants and harbors peptides for vaccine development.Immunobiology. 2021 Sep;226(5):152091. doi: 10.1016/j.imbio.2021.152091. Epub 2021 Jul 13. Immunobiology. 2021. PMID: 34303920 Free PMC article.
-
The Fight against COVID-19 on the Multi-Protease Front and Surroundings: Could an Early Therapeutic Approach with Repositioning Drugs Prevent the Disease Severity?Biomedicines. 2021 Jun 23;9(7):710. doi: 10.3390/biomedicines9070710. Biomedicines. 2021. PMID: 34201505 Free PMC article. Review.
-
The molecular mechanism of SARS-CoV-2 evading host antiviral innate immunity.Virol J. 2022 Mar 19;19(1):49. doi: 10.1186/s12985-022-01783-5. Virol J. 2022. PMID: 35305698 Free PMC article. Review.
-
Effects of nutrients on immunomodulation in patients with severe COVID-19: Current knowledge.World J Crit Care Med. 2022 Jul 9;11(4):201-218. doi: 10.5492/wjccm.v11.i4.201. eCollection 2022 Jul 9. World J Crit Care Med. 2022. PMID: 36051942 Free PMC article. Review.
-
SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target.Cell Res. 2021 Aug;31(8):847-860. doi: 10.1038/s41422-021-00519-4. Epub 2021 Jun 10. Cell Res. 2021. PMID: 34112954 Free PMC article.
References
-
- Annes J.P., Rifkin D.B., Munger J.S. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett. 2002;511:65–68. PMID:11821050. - PubMed
-
- Arcangeli A., Becchetti A. Integrin structure and functional relation with ion channels. Adv. Exp. Med. Biol. 2010;674:1–7. - PubMed
-
- Asmus M.J., Barros M.D., Liang J., Chesrown S.E., Hendeles L. Pulmonary function response to EDTA, an additive in nebulized bronchodilators. J. Allergy Clin. Immunol. 2001;107:68–72. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

