The Impact of Tiered-Pricing Framework on Generic Entry in Canada
- PMID: 33233033
- PMCID: PMC9309918
- DOI: 10.34172/ijhpm.2020.215
The Impact of Tiered-Pricing Framework on Generic Entry in Canada
Abstract
Background: Generic drug prices have been capped at specified percentages of the interchangeable branded drug's price by the Canadian provincial public drug plans since 1993. The Pan-Canadian Pharmaceutical Alliance, formed as a coalition by the provinces/territories in Canada, implemented an alternative approach, a tiered-pricing framework (TPF) for new generic drugs on April 1, 2014, under which the percentage varies with the number of generic firms in each market. We evaluate the impact of the TPF on generic entry, ie, listing in public drug plans in Canada.
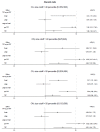
Methods: Our study compared the pre-TPF period (01/01/2012-03/31/2014) with the TPF period (04/01/2014- 06/30/2016). Prescription drugs from nine provincial public drug plans were grouped into a "market" if they had the same active ingredient and strength, route of administration, and dosage form. Each "market" was contestable by generics and met the eligibility criteria for TPF. At the "market" level, Cox proportional-hazards models with time-varying covariates were used to measure the impact of the TPF on the first generic listing in any provincial public drug plan in Canada relative to the first launch date worldwide.
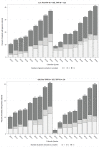
Results: A total of 189 markets in Canada were selected for the analyses. Generic drugs in small markets were more likely to be listed in Canada during the TPF period compared to the pre-TPF period (hazard ratio [HR], 95% CI: 3.81, 1.51-9.62). There was no significant difference in generic drug listings in large markets between the two policy periods.
Conclusion: TPF speeds up generic entry in small markets and generates the benefits of generic competition while avoiding the pitfalls of the previously employed price-cap regulations.
Keywords: Canada; Generic Drug; Generic Entry; Price-Cap Regulation; Tiered-Pricing Framework.
© 2022 The Author(s); Published by Kerman University of Medical Sciences This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
-
- Canadian Institute for Health Information (CIHI). Prescribed Drug Spending in Canada, 2018: A Focus on Public Drug Programs. Ottawa: CIHI; 2018.
-
- Douglas K, Jutras C. Patent Protection for Pharmaceutical Products in Canada: Chronology of Significant Events. Ottawa: Parliament of Canada; 2008.
-
- Government of Canada FAT and DC. United States-Mexico-Canada Agreement (USMCA) - Intellectual property chapter summary. GAC. http://international.gc.ca/trade-commerce/trade-agreements-accords-comme.... Accessed October 24, 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous