Synthesis of Polycyclic Ether-Benzopyrans and In Vitro Inhibitory Activity against Leishmania tarentolae
- PMID: 33233418
- PMCID: PMC7700287
- DOI: 10.3390/molecules25225461
Synthesis of Polycyclic Ether-Benzopyrans and In Vitro Inhibitory Activity against Leishmania tarentolae
Abstract
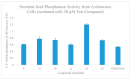
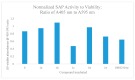
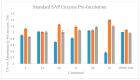
Construction of a focused library of polycyclic ether-benzopyrans was undertaken in order to discover new therapeutic compounds that affect Leishmania growth and infectivity. This is especially of interest since there are few drug therapies for leishmaniasis that do not have serious drawbacks such high cost, side effects, and emerging drug resistance. The construction of these polycyclic ether-benzopyrans utilized an acetoxypyranone-alkene [5+2] cycloaddition and the Suzuki-Miyaura cross-coupling. The multi-gram quantity of the requisite aryl bromide was obtained followed by effective Pd-catalyzed coupling with boronic acid derivatives. Compounds were tested in vitro using the parasitic protozoan, Leishmania tarentolae. Effects of concentration, time, and exposure to light were evaluated. In addition, the effects on secreted acid phosphatase activity and nitric oxide production were investigated, since both have been implicated in parasite infectivity. The data presented herein are indicative of disruption of the Leishmania tarentolae and thus provide impetus for the development and testing of a more extensive library.
Keywords: Leishmania; Suzuki-Miyaura; benzopyran; cycloaddition; nitric oxide; oxabicyclo[3.2.1]-octane; oxidopyrylium; secreted acid phosphatase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
A domino Knoevenagel hetero-Diels-Alder reaction for the synthesis of polycyclic chromene derivatives and evaluation of their cytotoxicity.Bioorg Med Chem Lett. 2012 Mar 1;22(5):1995-9. doi: 10.1016/j.bmcl.2012.01.033. Epub 2012 Jan 21. Bioorg Med Chem Lett. 2012. PMID: 22330634
-
Synthesis of polycyclic isoindoline derivatives via tandem Pd-catalyzed coupling, propargyl-allenyl isomerization, [4 + 2] cycloaddition and aromatization reaction.J Org Chem. 2012 Nov 16;77(22):10409-15. doi: 10.1021/jo301437k. Epub 2012 Nov 8. J Org Chem. 2012. PMID: 23051023
-
Stereocontrolled synthesis of (E)-stilbene derivatives by palladium-catalyzed Suzuki-Miyaura cross-coupling reaction.Bioorg Med Chem Lett. 2018 Sep 1;28(16):2693-2696. doi: 10.1016/j.bmcl.2018.04.004. Epub 2018 Apr 3. Bioorg Med Chem Lett. 2018. PMID: 29685657
-
Generation of cyclopenta[c]chromenes via a palladium-catalyzed reaction of 2-alkynylphenol with 2-alkynylvinyl bromide.Chem Commun (Camb). 2012 Jun 7;48(45):5581-3. doi: 10.1039/c2cc32077k. Epub 2012 May 1. Chem Commun (Camb). 2012. PMID: 22547176
-
Leishmania spp.: proficiency of drug-resistant parasites.Int J Antimicrob Agents. 2007 Jun;29(6):637-42. doi: 10.1016/j.ijantimicag.2007.01.004. Epub 2007 Mar 13. Int J Antimicrob Agents. 2007. PMID: 17353113 Review.
References
-
- Centers for Disease Control and Prevention (CDC) About Leishmaniasis. [(accessed on 17 October 2020)]; Available online: https://www.cdc.gov/parasites/leishmaniasis/gen_info/faqs.html.
-
- Centers for Disease Control and Prevention (CDC) Epidemiology and Risk Factors. [(accessed on 17 October 2020)]; Available online: https://www.cdc.gov/parasites/leishmaniasis/epi.html.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

