A Revised Protocol for Culture of Airway Epithelial Cells as a Diagnostic Tool for Primary Ciliary Dyskinesia
- PMID: 33233428
- PMCID: PMC7700393
- DOI: 10.3390/jcm9113753
A Revised Protocol for Culture of Airway Epithelial Cells as a Diagnostic Tool for Primary Ciliary Dyskinesia
Abstract
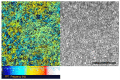
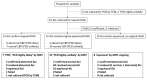
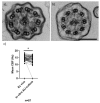
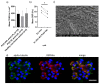
Air-liquid interface (ALI) culture of nasal epithelial cells is a valuable tool in the diagnosis and research of primary ciliary dyskinesia (PCD). Ex vivo samples often display secondary dyskinesia from cell damage during sampling, infection or inflammation confounding PCD diagnostic results. ALI culture enables regeneration of healthy cilia facilitating differentiation of primary from secondary ciliary dyskinesia. We describe a revised ALI culture method adopted from April 2018 across three collaborating PCD diagnostic sites, including current University Hospital Southampton COVID-19 risk mitigation measures, and present results. Two hundred and forty nasal epithelial cell samples were seeded for ALI culture and 199 (82.9%) were ciliated. Fifty-four of 83 (63.9%) ex vivo samples which were originally equivocal or insufficient provided diagnostic information following in vitro culture. Surplus basal epithelial cells from 181 nasal brushing samples were frozen in liquid nitrogen; 39 samples were ALI-cultured after cryostorage and all ciliated. The ciliary beat patterns of ex vivo samples (by high-speed video microscopy) were recapitulated, scanning electron microscopy demonstrated excellent ciliation, and cilia could be immuno-fluorescently labelled (anti-alpha-tubulin and anti-RSPH4a) in representative cases that were ALI-cultured after cryostorage. In summary, our ALI culture protocol provides high ciliation rates across three centres, minimising patient recall for repeat brushing biopsies and improving diagnostic certainty. Cryostorage of surplus diagnostic samples was successful, facilitating PCD research.
Keywords: ALI culture; PCD; bio-resource; diagnostics; primary nasal epithelium.
Conflict of interest statement
All authors certify that they have no conflicts of interest to declare.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

