Sinapic Acid Inhibits Cardiac Hypertrophy via Activation of Mitochondrial Sirt3/SOD2 Signaling in Neonatal Rat Cardiomyocytes
- PMID: 33233476
- PMCID: PMC7700612
- DOI: 10.3390/antiox9111163
Sinapic Acid Inhibits Cardiac Hypertrophy via Activation of Mitochondrial Sirt3/SOD2 Signaling in Neonatal Rat Cardiomyocytes
Abstract
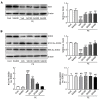
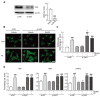
Sinapic acid (SA) is a naturally occurring phenolic compound with antioxidant properties. It also has a wide range of pharmacological properties, such as anti-inflammatory, anticancer, and hepatoprotective properties. The present study aimed to evaluate the potential pharmacological effects of SA against hypertrophic responses in neonatal rat cardiomyocytes. In order to evaluate the preventive effect of SA on cardiac hypertrophy, phenylephrine (PE)-induced hypertrophic cardiomyocytes were treated with subcytotoxic concentrations of SA. SA effectively suppressed hypertrophic responses, such as cell size enlargement, sarcomeric rearrangement, and fetal gene re-expression. In addition, SA significantly inhibited the expression of mitogen-activated protein kinase (MAPK) proteins as pro-hypertrophic factors and protected the mitochondrial functions from hypertrophic stimuli. Notably, SA activated Sirt3, a mitochondrial deacetylase, and SOD2, a mitochondrial antioxidant, in hypertrophic cardiomyocytes. SA also inhibited oxidative stress in hypertrophic cardiomyocytes. However, the protective effect of SA was significantly reduced in Sirt3-silenced hypertrophic cardiomyocytes, indicating that SA exerts its beneficial effect through Sirt3/SOD signaling. In summary, this is the first study to reveal the potential pharmacological action and inhibitory mechanism of SA as an antioxidant against cardiac hypertrophy, suggesting that SA could be utilized for the treatment of cardiac hypertrophy.
Keywords: SOD2; Sirt3; antioxidant; cardiac hypertrophy; oxidative stress; sinapic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Frey N., Katus H.A., Olson E.N., Hill J.A. Hypertrophy of the heart: A new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. - DOI - PubMed
-
- Katz A.M. Cardiomyopathy of overload. A major determinant of prognosis in congestive heart failure. N. Engl. J. Med. 1990;322:100–110. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

