Dynamic Regulation of Peroxisomes and Mitochondria during Fungal Development
- PMID: 33233491
- PMCID: PMC7711908
- DOI: 10.3390/jof6040302
Dynamic Regulation of Peroxisomes and Mitochondria during Fungal Development
Abstract
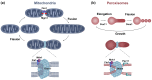
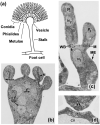
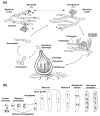
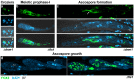
Peroxisomes and mitochondria are organelles that perform major functions in the cell and whose activity is very closely associated. In fungi, the function of these organelles is critical for many developmental processes. Recent studies have disclosed that, additionally, fungal development comprises a dynamic regulation of the activity of these organelles, which involves a developmental regulation of organelle assembly, as well as a dynamic modulation of the abundance, distribution, and morphology of these organelles. Furthermore, for many of these processes, the dynamics of peroxisomes and mitochondria are governed by common factors. Notably, intense research has revealed that the process that drives the division of mitochondria and peroxisomes contributes to several developmental processes-including the formation of asexual spores, the differentiation of infective structures by pathogenic fungi, and sexual development-and that these processes rely on selective removal of these organelles via autophagy. Furthermore, evidence has been obtained suggesting a coordinated regulation of organelle assembly and dynamics during development and supporting the existence of regulatory systems controlling fungal development in response to mitochondrial activity. Gathered information underscores an important role for mitochondrial and peroxisome dynamics in fungal development and suggests that this process involves the concerted activity of these organelles.
Keywords: cell differentiation; fungi; mitochondria; mitochondrial fission; mitophagy; organelle dynamics; peroxisome; peroxisome fission; pexophagy; sexual development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Spatiotemporal Dynamic Regulation of Organelles During Meiotic Development, Insights From Fungi.Front Cell Dev Biol. 2022 Apr 25;10:886710. doi: 10.3389/fcell.2022.886710. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35547805 Free PMC article.
-
Distinct Contributions of the Peroxisome-Mitochondria Fission Machinery During Sexual Development of the Fungus Podospora anserina.Front Microbiol. 2020 Apr 15;11:640. doi: 10.3389/fmicb.2020.00640. eCollection 2020. Front Microbiol. 2020. PMID: 32351478 Free PMC article.
-
Peroxisome dynamics during development of the fungus Podospora anserina.Mycologia. 2016 May-Jun;108(3):590-602. doi: 10.3852/15-112. Epub 2016 Feb 23. Mycologia. 2016. PMID: 26908647
-
Peroxisomes and sexual development in fungi.Front Physiol. 2013 Sep 6;4:244. doi: 10.3389/fphys.2013.00244. Front Physiol. 2013. PMID: 24046747 Free PMC article. Review.
-
Fission Impossible (?)-New Insights into Disorders of Peroxisome Dynamics.Cells. 2022 Jun 14;11(12):1922. doi: 10.3390/cells11121922. Cells. 2022. PMID: 35741050 Free PMC article. Review.
Cited by
-
AoBck1 and AoMkk1 Are Necessary to Maintain Cell Wall Integrity, Vegetative Growth, Conidiation, Stress Resistance, and Pathogenicity in the Nematode-Trapping Fungus Arthrobotrys oligospora.Front Microbiol. 2021 Jun 22;12:649582. doi: 10.3389/fmicb.2021.649582. eCollection 2021. Front Microbiol. 2021. PMID: 34239505 Free PMC article.
-
The Glyoxysomal Protease LON2 Is Involved in Fruiting-Body Development, Ascosporogenesis and Stress Resistance in Sordaria macrospora.J Fungi (Basel). 2021 Jan 26;7(2):82. doi: 10.3390/jof7020082. J Fungi (Basel). 2021. PMID: 33530609 Free PMC article.
-
What are the 100 most cited fungal genera?Stud Mycol. 2024 Jul;108:1-411. doi: 10.3114/sim.2024.108.01. Epub 2024 Jul 15. Stud Mycol. 2024. PMID: 39100921 Free PMC article.
-
Spatiotemporal Dynamic Regulation of Organelles During Meiotic Development, Insights From Fungi.Front Cell Dev Biol. 2022 Apr 25;10:886710. doi: 10.3389/fcell.2022.886710. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35547805 Free PMC article.
-
The essential role of fungal peroxisomes in plant infection.Mol Plant Pathol. 2022 Jun;23(6):781-794. doi: 10.1111/mpp.13180. Epub 2022 Jan 10. Mol Plant Pathol. 2022. PMID: 35001508 Free PMC article. Review.
References
-
- Shai N., Yifrach E., van Roermund C.W.T., Cohen N., Bibi C., IJlst L., Cavellini L., Meurisse J., Schuster R., Zada L., et al. Systematic mapping of contact sites reveals tethers and a function for the peroxisome-mitochondria contact. Nat. Commun. 2018;9:1761. doi: 10.1038/s41467-018-03957-8. - DOI - PMC - PubMed
-
- Mattiazzi Usaj M., Brloznik M., Kaferle P., Zitnik M., Wolinski H., Leitner F., Kohlwein S.D., Zupan B., Petrovic U. Genome-Wide Localization Study of Yeast Pex11 Identifies Peroxisome-Mitochondria Interactions through the ERMES Complex. J. Mol. Biol. 2015;427:2072–2087. doi: 10.1016/j.jmb.2015.03.004. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

