Effect of High-Dose vs Standard-Dose Vitamin D3 Supplementation on Body Composition among Patients with Advanced or Metastatic Colorectal Cancer: A Randomized Trial
- PMID: 33233566
- PMCID: PMC7699725
- DOI: 10.3390/cancers12113451
Effect of High-Dose vs Standard-Dose Vitamin D3 Supplementation on Body Composition among Patients with Advanced or Metastatic Colorectal Cancer: A Randomized Trial
Abstract
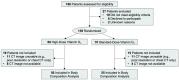
Skeletal muscle and adipose tissue express the vitamin D receptor and may be a mechanism through which vitamin D supplementation slows cancer progression and reduces cancer death. In this exploratory analysis of a double-blind, multicenter, randomized phase II clinical trial, 105 patients with advanced or metastatic colorectal cancer who were receiving chemotherapy were randomized to either high-dose vitamin D3 (4000 IU) or standard-dose (400 IU) vitamin D3. Body composition was measured with abdominal computed tomography at enrollment (baseline) and after cycle 8 of chemotherapy (16 weeks). As compared with standard-dose vitamin D3, high-dose vitamin D3 did not significantly change body weight [-0.7 kg; (95% CI: -3.5, 2.0)], body mass index [-0.2 kg/m2; (95% CI: -1.2, 0.7)], muscle area [-1.7 cm2; (95% CI: -9.6, 6.3)], muscle attenuation [-0.4 HU; (95% CI: -4.2, 3.2)], visceral adipose tissue area [-7.5 cm2; (95% CI: -24.5, 9.6)], or subcutaneous adipose tissue area [-8.3 cm2; (95% CI: -35.5, 18.9)] over the first 8 cycles of chemotherapy. Among patients with advanced or metastatic colorectal cancer, the addition of high-dose vitamin D3, vs standard-dose vitamin D3, to standard chemotherapy did not result in any changes in body composition.
Keywords: adipose tissue; cholecalciferol; colorectal neoplasms; mediation; prognosis; randomized; skeletal muscle.
Conflict of interest statement
J.C.B. reported receiving grants from the National Institutes of Health the American Institute for Cancer Research, and the Susan G. Komen Foundation. H.S.N. reported being employed at AbbVie. T.A.A. reported receiving grants from Eli Lilly and Bristol-Myers Squibb; and receiving personal fees from Bristol-Myers Squibb and Genentech. M.B.Y. reported consulting fees from Janssen Pharmaceuticals and receiving personal fees for peer review services from UpToDate. J.M.C. received research funding to his institution from Abbvie, Merus, Roche, and Bristol Myers Squib; received research funding from Merck, Astrazeneca, Esperas Pharma, and Tesaro; received consulting fees from Bristol Myers Squibb; and received travel funding from Bristol Myers Squib. D.S. reported receiving personal fees from JAMA; receiving grants from Pfizer, the Alliance for Clinical Trials in Oncology, the American Association for Cancer Research, the National Cancer Institute, the Patient-Centered Outcomes Research Institute, and the American Cancer Society; and receiving nonfinancial support from Epic Systems Corporation. A.J.B. reported receiving personal fees from Taiho Oncology, Bayer, Eisai, Exelixis, and Celgene. E.C. reported being employed at Amgen; and receiving funding from Dana-Farber Cancer Institute and owning shares of Amgen stock. J.A.C. reported receiving personal fees from Novartis, Ipsen, Lexicon, AAA, and Exelixis; and receiving support from Novartis, Merck, Sanofi, and Lilly. B.W. reported receiving grants from Celgene; and receiving personal fees from BioLineRx and Grail. C.A.T. reported receiving personal fees from Genetech/ Roche, Bristol-Myers Squibb, and Aztra Zeneca. H.Z. reported receiving grants from the National Institutes of Health. C.S.F. reported receiving personal fees from Eli Lilly, Entrinsic Health, Pfizer, Merck, Sanofi, Roche, Genentech, Merrimack Pharma, Dicerna, Bayer, Celgene, Agios, Gilead Sciences, Five Prime Therapeutics, Taiho, KEW, and CytomX Therapeutics; and receiving support from CytomX Therapeutics. K.N. reported receiving grants from the National Cancer Institute, Genentech, Consano, Gilead Sciences, Tarrex Biopharma, Trovagene, Celgene, and Pharmavite; and receiving personal fees from Genentech, Lilly, Tarrex Biopharma, Bayer, and Seattle Genetics. J.A.M. has received institutional research funding from Boston Biomedical, has served as an advisor/consultant to Ignyta and COTA Healthcare, and served on a grant review panel for the National Comprehensive Cancer Network funded by Taiho Pharmaceutical. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
-
- Schleicher R.L., Sternberg M.R., Lacher D.A., Sempos C.T., Looker A.C., Durazo-Arvizu R.A., Yetley E.A., Chaudhary-Webb M., Maw K.L., Pfeiffer C.M., et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am. J. Clin. Nutr. 2016;104:454–461. doi: 10.3945/ajcn.115.127985. - DOI - PMC - PubMed
-
- Michaelsson K., Baron J.A., Snellman G., Gedeborg R., Byberg L., Sundstrom J., Berglund L., Arnlov J., Hellman P., Blomhoff R., et al. Plasma vitamin D and mortality in older men: A community-based prospective cohort study. Am. J. Clin. Nutr. 2010;92:841–848. doi: 10.3945/ajcn.2010.29749. - DOI - PubMed
Grants and funding
- P50CA127003/CA/NCI NIH HHS/United States
- P30-DK072476/DK/NIDDK NIH HHS/United States
- R25-CA203650/CA/NCI NIH HHS/United States
- P30 DK072476/DK/NIDDK NIH HHS/United States
- R00 CA218603/CA/NCI NIH HHS/United States
- U54 GM104940/GM/NIGMS NIH HHS/United States
- R01 CA205406/CA/NCI NIH HHS/United States
- R01 CA169141/CA/NCI NIH HHS/United States
- R01CA118553/CA/NCI NIH HHS/United States
- U54-GM104940/National Institute of General Medicine Sciences
- R01CA205406/CA/NCI NIH HHS/United States
- R00-CA218603/CA/NCI NIH HHS/United States
- R01 CA118553/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials