Microbial Upgrading of Acetate into Value-Added Products-Examining Microbial Diversity, Bioenergetic Constraints and Metabolic Engineering Approaches
- PMID: 33233586
- PMCID: PMC7699770
- DOI: 10.3390/ijms21228777
Microbial Upgrading of Acetate into Value-Added Products-Examining Microbial Diversity, Bioenergetic Constraints and Metabolic Engineering Approaches
Abstract
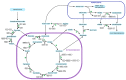
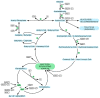
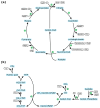
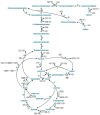
Ecological concerns have recently led to the increasing trend to upgrade carbon contained in waste streams into valuable chemicals. One of these components is acetate. Its microbial upgrading is possible in various species, with Escherichia coli being the best-studied. Several chemicals derived from acetate have already been successfully produced in E. coli on a laboratory scale, including acetone, itaconic acid, mevalonate, and tyrosine. As acetate is a carbon source with a low energy content compared to glucose or glycerol, energy- and redox-balancing plays an important role in acetate-based growth and production. In addition to the energetic challenges, acetate has an inhibitory effect on microorganisms, reducing growth rates, and limiting product concentrations. Moreover, extensive metabolic engineering is necessary to obtain a broad range of acetate-based products. In this review, we illustrate some of the necessary energetic considerations to establish robust production processes by presenting calculations of maximum theoretical product and carbon yields. Moreover, different strategies to deal with energetic and metabolic challenges are presented. Finally, we summarize ways to alleviate acetate toxicity and give an overview of process engineering measures that enable sustainable acetate-based production of value-added chemicals.
Keywords: Escherichia coli; acetate; acetate metabolism; acetate tolerance; acetate-derived chemicals; bioenergetic constraints; metabolic engineering; process engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Brown S.W., Meyer H.-P., Fiechter A. Continuous production of human leukocyte interferon with Escherichia coli and continuous cell lysis in a two stage chemostat. Appl. Microbiol. Biotechnol. 1985;23:5–9. doi: 10.1007/BF02660110. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

