The Immune Functions of Keratinocytes in Skin Wound Healing
- PMID: 33233704
- PMCID: PMC7699912
- DOI: 10.3390/ijms21228790
The Immune Functions of Keratinocytes in Skin Wound Healing
Abstract
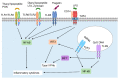
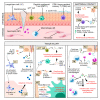
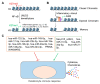
As the most dominant cell type in the skin, keratinocytes play critical roles in wound repair not only as structural cells but also exerting important immune functions. This review focuses on the communications between keratinocytes and immune cells in wound healing, which are mediated by various cytokines, chemokines, and extracellular vesicles. Keratinocytes can also directly interact with T cells via antigen presentation. Moreover, keratinocytes produce antimicrobial peptides that can directly kill the invading pathogens and contribute to wound repair in many aspects. We also reviewed the epigenetic mechanisms known to regulate keratinocyte immune functions, including histone modifications, non-protein-coding RNAs (e.g., microRNAs, and long noncoding RNAs), and chromatin dynamics. Lastly, we summarized the current evidence on the dysregulated immune functions of keratinocytes in chronic nonhealing wounds. Based on their crucial immune functions in skin wound healing, we propose that keratinocytes significantly contribute to the pathogenesis of chronic wound inflammation. We hope this review will trigger an interest in investigating the immune roles of keratinocytes in chronic wound pathology, which may open up new avenues for developing innovative wound treatments.
Keywords: antimicrobial peptide; chronic wounds; cytokine; epigenetic regulation; immune function; inflammation; keratinocyte; long noncoding RNA; microRNA; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Isaac C., Paggiaro A.O., Aldunate J.L.C.B., Herson M.R., Altran S.C., Mônica Beatriz M., Ferreira M.C. Role of keratinocytes in wound contraction: An impact assessment using a model of collagen matrix populated with fibroblasts. Rev. Bras. Cir. Plást. 2011;26:402–406. doi: 10.1590/S1983-51752011000300007. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

