Anaplasma and Theileria Pathogens in Cattle of Lambwe Valley, Kenya: A Case for Pro-Active Surveillance in the Wildlife-Livestock Interface
- PMID: 33233713
- PMCID: PMC7699859
- DOI: 10.3390/microorganisms8111830
Anaplasma and Theileria Pathogens in Cattle of Lambwe Valley, Kenya: A Case for Pro-Active Surveillance in the Wildlife-Livestock Interface
Abstract
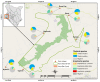
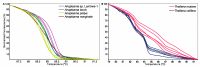
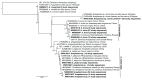
Tick-borne pathogens (TBPs) are major constraints to livestock production and a threat to public health in Africa. This cross-sectional study investigated the risk of infection with TBPs in cattle of Lambwe Valley, Kenya. Blood samples of 680 zebu cattle from 95 herds in six geospatial clusters within 5 km of Ruma National Park were screened for bacterial and protozoan TBPs by high-resolution melting analysis and sequencing of PCR products. We detected Anaplasma bovis (17.4%), Anaplasma platys (16.9%), Anaplasma marginale (0.6%), Theileria velifera (40%), and Theileria mutans (25.7%), as well as an Anaplasma sp. (11.6%) that matched recently reported Anaplasma sp. sequences from Ethiopia. Babesia, Rickettsia, and Ehrlichia spp. were not detected. The animal and herd-level prevalences for TBPs were 78.5% (95% confidence intervals (CI): 75.3, 81.5) and 95.8% (95% CI: 91.8, 99.8), respectively. About 31.6% of cattle were co-infected with 13 combinations of TBPs. The prevalence of TBPs differed between clusters and age, but the risk of infection was not associated with sex, herd size, or the distance of homesteads from Ruma. This study adds insight into the epidemiology of TBPs around Ruma and highlights the need for proactive surveillance of TBPs in livestock-wildlife interfaces.
Keywords: Anaplasma; Kenya; Theileria; tick-borne pathogens; wildlife–livestock interface; zebu cattle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Molecular prevalence and risk factors associated with tick-borne pathogens in cattle in western Kenya.BMC Vet Res. 2021 Nov 27;17(1):363. doi: 10.1186/s12917-021-03074-7. BMC Vet Res. 2021. PMID: 34838023 Free PMC article.
-
Epidemiology of tick-borne pathogens of cattle and tick control practices in coastal Kenya.Prev Vet Med. 2022 Dec;209:105777. doi: 10.1016/j.prevetmed.2022.105777. Epub 2022 Oct 11. Prev Vet Med. 2022. PMID: 36272258
-
Molecular detection of selected tick-borne pathogens infecting cattle at the wildlife-livestock interface of Queen Elizabeth National Park in Kasese District, Uganda.Ticks Tick Borne Dis. 2021 Sep;12(5):101772. doi: 10.1016/j.ttbdis.2021.101772. Epub 2021 Jun 17. Ticks Tick Borne Dis. 2021. PMID: 34214889
-
Global prevalence and species diversity of tick-borne pathogens in buffaloes worldwide: a systematic review and meta-analysis.Parasit Vectors. 2023 Mar 30;16(1):115. doi: 10.1186/s13071-023-05727-y. Parasit Vectors. 2023. PMID: 36998029 Free PMC article.
-
Tick-borne diseases in ruminants of Central and Southern Italy: epidemiology and case reports.Parassitologia. 1999 Sep;41 Suppl 1:95-100. Parassitologia. 1999. PMID: 11071553 Review.
Cited by
-
Molecular prevalence and risk factors associated with tick-borne pathogens in cattle in western Kenya.BMC Vet Res. 2021 Nov 27;17(1):363. doi: 10.1186/s12917-021-03074-7. BMC Vet Res. 2021. PMID: 34838023 Free PMC article.
-
Babesia bigemina and Theileria annulata infections in cattle: molecular detection, phylogenetic analysis, and assessment of risk factors.Trop Anim Health Prod. 2024 Sep 26;56(8):282. doi: 10.1007/s11250-024-04122-8. Trop Anim Health Prod. 2024. PMID: 39322769
-
Distribution and Prevalence of Ticks and Tick-Borne Pathogens at the Wildlife-Livestock Interface in Africa: A Systematic Review.Vet Sci. 2025 Apr 13;12(4):364. doi: 10.3390/vetsci12040364. Vet Sci. 2025. PMID: 40284866 Free PMC article. Review.
-
Prevalence of Trypanosome Species in Cattle Near Ruma National Park, Lambwe Valley, Kenya: An Update From the Historical Focus for African Trypanosomosis.Front Vet Sci. 2021 Nov 2;8:750169. doi: 10.3389/fvets.2021.750169. eCollection 2021. Front Vet Sci. 2021. PMID: 34796227 Free PMC article.
-
Anaplasma Species in Africa-A Century of Discovery: A Review on Molecular Epidemiology, Genetic Diversity, and Control.Pathogens. 2023 May 12;12(5):702. doi: 10.3390/pathogens12050702. Pathogens. 2023. PMID: 37242372 Free PMC article. Review.
References
-
- Mwamuye M.M., Kariuki E., Omondi D., Kabii J., Odongo D., Masiga D., Villinger J. Novel Rickettsia and emergent tick-borne pathogens: A molecular survey of ticks and tick-borne pathogens in Shimba Hills National Reserve, Kenya. Ticks Tick Borne Dis. 2017;8:208–218. doi: 10.1016/j.ttbdis.2016.09.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

