Detailed Insight into the Interaction of Bicyclic Somatostatin Analogue with Cu(II) Ions
- PMID: 33233719
- PMCID: PMC7699899
- DOI: 10.3390/ijms21228794
Detailed Insight into the Interaction of Bicyclic Somatostatin Analogue with Cu(II) Ions
Abstract
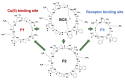
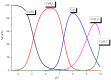
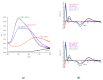
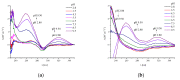
Somatostatin analogues are useful pharmaceuticals in peptide receptor radionuclide therapy. In previous studies, we analyzed a new bicyclic somatostatin analogue (BCS) in connection with Cu(II) ions. Two characteristic sites were present in the peptide chain: the receptor- and the metal-binding site. We have already shown that this ligand can form very stable imidazole complexes with the metal ion. In this work, our aim was to characterize the intramolecular interaction that occurs in the peptide molecule. Therefore, we analyzed the coordination abilities of two cyclic ligands, i.e., P1 only with the metal binding site and P2 with both sites, but without the disulfide bond. Furthermore, we used magnetic circular dichroism (MCD) spectroscopy to better understand the coordination process. We applied this method to analyze spectra of P1, P2, and BCS, which we have described previously. Additionally, we analyzed the MCD spectra of P3 ligand, which has only the receptor binding site in its structure. We have unequivocally shown that the presence of the Phe-Trp-Lys-Thr motif and the disulfide bond significantly increases the metal binding efficiency.
Keywords: bicyclic peptide; copper(II) complexes; magnetic circular dichroism; potentiometric titration; somatostatin analogues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Novel short-chain analogues of somatostatin as ligands for Cu(II) ions. Role of the metal ion binding on the spatial structure of the ligand.J Inorg Biochem. 2012 Dec;117:10-7. doi: 10.1016/j.jinorgbio.2012.08.023. Epub 2012 Sep 7. J Inorg Biochem. 2012. PMID: 23078770
-
The Binding Ability of a Bicyclic Somatostatin Analogue Towards Cu(II) Ions.Chem Biodivers. 2020 Aug;17(8):e2000307. doi: 10.1002/cbdv.202000307. Epub 2020 Jul 20. Chem Biodivers. 2020. PMID: 32470208
-
Coordination of copper(II) ions by the fragments of neuropeptide gamma containing D1, H9, H12 residues and products of copper-catalyzed oxidation.Dalton Trans. 2012 Feb 14;41(6):1683-94. doi: 10.1039/c1dt10592b. Epub 2011 Dec 13. Dalton Trans. 2012. PMID: 22159001
-
The Role of the Disulfide Bridge in the Copper (II) Binding by the Cyclic His4-Peptide.Int J Mol Sci. 2021 Jun 16;22(12):6458. doi: 10.3390/ijms22126458. Int J Mol Sci. 2021. PMID: 34208680 Free PMC article.
-
The coordination abilities of the multiHis-cyclopeptide with two metal-binding centers--potentiometric and spectroscopic investigation.Dalton Trans. 2012 Oct 21;41(39):12114-20. doi: 10.1039/c2dt31224g. Dalton Trans. 2012. PMID: 22918544
Cited by
-
Cu(Proline)2 Complex: A Model of Bio-Copper Structural Ambivalence.Molecules. 2022 Sep 9;27(18):5846. doi: 10.3390/molecules27185846. Molecules. 2022. PMID: 36144582 Free PMC article.
-
The Role of the Unbinding Cycle on the Coordination Abilities of the Bi-Cyclopeptides toward Cu(II) Ions.Molecules. 2024 May 8;29(10):2197. doi: 10.3390/molecules29102197. Molecules. 2024. PMID: 38792059 Free PMC article.
References
-
- Eder M., Pavan S., Bauder-Wüst U., van Rietschoten K., Baranski A.-C., Harrison H., Campbell S., Stace C.L., Walker E.H., Chen L., et al. Bicyclic peptides as a new modality for imaging and targeting of proteins overexpressed by tumors. Cancer Res. 2019;79:841–852. doi: 10.1158/0008-5472.CAN-18-0238. - DOI - PubMed
-
- Falb E., Salitra Y., Yechezkel T., Bracha M., Litman P., Olender R., Rosenfeld R., Senderowitz H., Jiang S., Goodman M. A bicyclic and hsst2 selective somatostatin analogue: Design, synthesis, conformational analysis and binding. Bioorg. Med. Chem. 2001;9:3255–3264. doi: 10.1016/S0968-0896(01)00234-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

