Rbm38 Reduces the Transcription Elongation Defect of the SMEK2 Gene Caused by Splicing Deficiency
- PMID: 33233740
- PMCID: PMC7699959
- DOI: 10.3390/ijms21228799
Rbm38 Reduces the Transcription Elongation Defect of the SMEK2 Gene Caused by Splicing Deficiency
Abstract
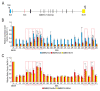
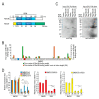
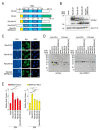
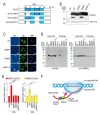
Pre-mRNA splicing is an essential mechanism for ensuring integrity of the transcriptome in eukaryotes. Therefore, splicing deficiency might cause a decrease in functional proteins and the production of nonfunctional, aberrant proteins. To prevent the production of such aberrant proteins, eukaryotic cells have several mRNA quality control mechanisms. In addition to the known mechanisms, we previously found that transcription elongation is attenuated to prevent the accumulation of pre-mRNA under splicing-deficient conditions. However, the detailed molecular mechanism behind the defect in transcription elongation remains unknown. Here, we showed that the RNA binding protein Rbm38 reduced the transcription elongation defect of the SMEK2 gene caused by splicing deficiency. This reduction was shown to require the N- and C-terminal regions of Rbm38, along with an important role being played by the RNA-recognition motif of Rbm38. These findings advance our understanding of the molecular mechanism of the transcription elongation defect caused by splicing deficiency.
Keywords: Rbm38; pre-mRNA splicing; spliceostatin A; transcription elongation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kaida D., Motoyoshi H., Tashiro E., Nojima T., Hagiwara M., Ishigami K., Watanabe H., Kitahara T., Yoshida T., Nakajima H., et al. Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat. Chem. Biol. 2007;3:576–583. doi: 10.1038/nchembio.2007.18. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

