Regulation of GABAARs by Transmembrane Accessory Proteins
- PMID: 33234346
- PMCID: PMC7855156
- DOI: 10.1016/j.tins.2020.10.011
Regulation of GABAARs by Transmembrane Accessory Proteins
Abstract
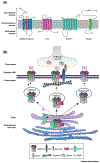
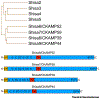
The vast majority of fast inhibitory transmission in the brain is mediated by GABA acting on GABAA receptors (GABAARs), which provides inhibitory balance to excitatory drive and controls neuronal output. GABAARs are also effectively targeted by clinically important drugs for treatment in a number of neurological disorders. It has long been hypothesized that function and pharmacology of GABAARs are determined by the channel pore-forming subunits. However, recent studies have provided new dimensions in studying GABAARs due to several transmembrane proteins that interact with GABAARs and modulate their trafficking and function. In this review, we summarize recent findings on these novel GABAAR transmembrane regulators and highlight a potential avenue to develop new GABAAR psychopharmacology by targeting these receptor-associated membrane proteins.
Keywords: Clptm1; GABA(A) receptor; GARLH4; Lhfpl4; Shisa7; auxiliary subunit; benzodiazepines.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

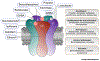
References
-
- Sieghart W and Savić MM (2018) International Union of Basic and Clinical Pharmacology. CVI: GABAA receptor subtype- and function-selective ligands: key issues in translation to humans. Pharmacol. Rev, 70, 836–878 - PubMed
-
- Thomson AM and Jovanovic JN (2010) Mechanisms underlying synapse-specific clustering of GABAA receptors. Eur. J. Neurosci 31, 2193–2203 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

