Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma
- PMID: 33234602
- PMCID: PMC7689089
- DOI: 10.1136/jitc-2020-001435
Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma
Abstract
Background and purpose: Combining inhibitors of vascular endothelial growth factor and the programmed cell death protein 1 (PD1) pathway has shown efficacy in multiple cancers, but the disease-specific and agent-specific mechanisms of benefit remain unclear. We examined the efficacy and defined the mechanisms of benefit when combining regorafenib (a multikinase antivascular endothelial growth factor receptor inhibitor) with PD1 blockade in murine hepatocellular carcinoma (HCC) models.
Basic procedures: We used orthotopic models of HCC in mice with liver damage to test the effects of regorafenib-dosed orally at 5, 10 or 20 mg/kg daily-combined with anti-PD1 antibodies (10 mg/kg intraperitoneally thrice weekly). We evaluated the effects of therapy on tumor vasculature and immune microenvironment using immunofluorescence, flow cytometry, RNA-sequencing, ELISA and pharmacokinetic/pharmacodynamic studies in mice and in tissue and blood samples from patients with cancer.
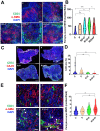
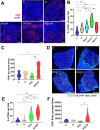
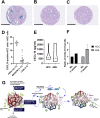
Main findings: Regorafenib/anti-PD1 combination therapy increased survival compared with regofarenib or anti-PD1 alone in a regorafenib dose-dependent manner. Combination therapy increased regorafenib uptake into the tumor tissues by normalizing the HCC vasculature and increasing CD8 T-cell infiltration and activation at an intermediate regorafenib dose. The efficacy of regorafenib/anti-PD1 therapy was compromised in mice lacking functional T cells (Rag1-deficient mice). Regorafenib treatment increased the transcription and protein expression of CXCL10-a ligand for CXCR3 expressed on tumor-infiltrating lymphocytes-in murine HCC and in blood of patients with HCC. Using Cxcr3-deficient mice, we demonstrate that CXCR3 mediated the increased intratumoral CD8 T-cell infiltration and the added survival benefit when regorafenib was combined with anti-PD1 therapy.
Principal conclusions: Judicious regorafenib/anti-PD1 combination therapy can inhibit tumor growth and increase survival by normalizing tumor vasculature and increasing intratumoral CXCR3+CD8 T-cell infiltration through elevated CXCL10 expression in HCC cells.
Keywords: combination; drug therapy; liver neoplasms; programmed cell death 1 receptor.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: LG reports personal fees from Agios Pharmaceuticals, Alentis Therapeutics, QED Therapeutics, H3 Biomedicine, Taiho Pharmaceuticals, Debiopharm, Incyte Corporation, SIRTEX and AstraZeneca. AXZ is a consultant/advisory board member for Bayer. RKJ received honorarium from Amgen and consultant fees from Chugai, Ophthotech, Merck, SPARC, SynDevRx and XTuit. Dr Jain owns equity in XTuit, Enlight, SPARC, SynDevRx and Accurius Therapeutics and serves as a paid member of the boards of trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund and Tekla World Healthcare Fund. He is a member of the scientific advisory board of Accurius Therapeutics. DZ, LF and the spouse of LLM are Bayer employees. MC is an AstraZeneca employee. DGD received consultant fees from Bayer, Simcere, Surface Oncology and Bristol Myers Squibb, and research grants from Bayer, Exelixis and Bristol Myers Squibb. No potential conflicts of interest were disclosed by other authors.
Figures



Similar articles
-
Immunomodulatory Effects of Lenvatinib Plus Anti-Programmed Cell Death Protein 1 in Mice and Rationale for Patient Enrichment in Hepatocellular Carcinoma.Hepatology. 2021 Nov;74(5):2652-2669. doi: 10.1002/hep.32023. Epub 2021 Sep 27. Hepatology. 2021. PMID: 34157147 Free PMC article.
-
Dual Programmed Death Receptor-1 and Vascular Endothelial Growth Factor Receptor-2 Blockade Promotes Vascular Normalization and Enhances Antitumor Immune Responses in Hepatocellular Carcinoma.Hepatology. 2020 Apr;71(4):1247-1261. doi: 10.1002/hep.30889. Epub 2019 Oct 14. Hepatology. 2020. PMID: 31378984 Free PMC article.
-
Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages.J Immunother Cancer. 2021 Mar;9(3):e001657. doi: 10.1136/jitc-2020-001657. J Immunother Cancer. 2021. PMID: 33753566 Free PMC article.
-
Regorafenib as treatment for patients with advanced hepatocellular cancer.Future Oncol. 2017 Oct;13(25):2223-2232. doi: 10.2217/fon-2017-0204. Epub 2017 Aug 2. Future Oncol. 2017. PMID: 28766967 Review.
-
Immunomodulatory Effects of Current Targeted Therapies on Hepatocellular Carcinoma: Implication for the Future of Immunotherapy.Semin Liver Dis. 2018 Nov;38(4):379-388. doi: 10.1055/s-0038-1673621. Epub 2018 Oct 24. Semin Liver Dis. 2018. PMID: 30357775
Cited by
-
Translational research on drug development and biomarker discovery for hepatocellular carcinoma.J Biomed Sci. 2024 Feb 17;31(1):22. doi: 10.1186/s12929-024-01011-y. J Biomed Sci. 2024. PMID: 38368324 Free PMC article. Review.
-
Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment.Nat Rev Drug Discov. 2022 Jun;21(6):440-462. doi: 10.1038/s41573-022-00415-5. Epub 2022 Mar 15. Nat Rev Drug Discov. 2022. PMID: 35292771 Review.
-
DFNA5 regulates immune cells infiltration and exhaustion.Cancer Cell Int. 2022 Mar 5;22(1):107. doi: 10.1186/s12935-022-02487-0. Cancer Cell Int. 2022. PMID: 35248047 Free PMC article.
-
Regorafenib combined with programmed cell death-1 inhibitor against refractory colorectal cancer and the platelet-to-lymphocyte ratio's prediction on effectiveness.World J Gastrointest Oncol. 2022 Apr 15;14(4):920-934. doi: 10.4251/wjgo.v14.i4.920. World J Gastrointest Oncol. 2022. PMID: 35582108 Free PMC article.
-
A tumor microenvironment-based classification of gastric cancer for more effective diagnosis and treatment.Res Sq [Preprint]. 2023 Aug 1:rs.3.rs-3089359. doi: 10.21203/rs.3.rs-3089359/v1. Res Sq. 2023. PMID: 37577519 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
