Clinicopathological characteristics and management of colitis with anti-PD1 immunotherapy alone or in combination with ipilimumab
- PMID: 33234603
- PMCID: PMC7689081
- DOI: 10.1136/jitc-2020-001488
Clinicopathological characteristics and management of colitis with anti-PD1 immunotherapy alone or in combination with ipilimumab
Abstract
Background: Colitis is one of the common immune-related adverse events that leads to morbidity and treatment discontinuation of immunotherapy. The clinical presentation, endoscopic and histopathological features and best management of this toxicity are not well defined.
Patients and methods: Patients with metastatic melanoma who received immunotherapy (programmed cell death protein 1 (PD1) antibodies, alone or in combination with a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibody (PD1 +CTLA-4)) and who developed clinically significant colitis (requiring systemic corticosteroids) were identified retrospectively from two academic centers. Clinical data were collected for all patients; endoscopic and histopathological data were examined in a subset.
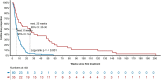
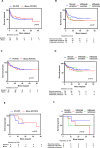
Results: From May 2013 to May 2019, 118/1507 (7.8%) patients developed significant colitis; 80/553 (14.5%) after PD1+CTLA-4, 35/1000 (3.5%) PD1 alone, and three patients after Ipilimumab (IPI) alone. Combination therapy-induced colitis was more frequent (14.5% vs 3.5% in PD1 alone, p=<0.0001), had an earlier onset (6.3 weeks vs 25.7 weeks, p=<0.001), was more severe (grade 3/4 69% vs 31%, p=<0.001), and are more likely to require higher doses of steroids (91% vs 74%, p=0.01) than PD1 colitis. Among all patients treated with steroids (N=114), 54 (47%) responded and required no further therapy (steroid sensitive), 47 patients (41%) responded to infliximab (infliximab sensitive), and 13 (11%) were infliximab refractory and needed further immunosuppressive drugs. Infliximab-refractory patients all had onset within 4 weeks of immunotherapy commencement and were more likely to have an underlying autoimmune disease, have higher grade colitis, and require longer immunosuppression, yet had similar response and survival than other patients with colitis. Of 43 (37%) patients re-resumed treatment with PD1 monotherapy after colitis resolution, 16 (37%) of whom developed recurrent colitis. Endoscopic and histopathologic data were available for 64 patients. Most had left-sided colitis, with an increase in chronic inflammatory cells and neutrophils within the lamina propria, an increase in neutrophils in the surface epithelium, without increased lymphocytes or increased eosinophils. Infliximab-refractory colitis had a trend towards more confluent pancolitis with edema, erythema, ulceration, and absent vascularity with neutrophilic infiltration and erosion.
Conclusion: Clinically significant colitis varies in presentation, response to immunosuppression, and endoscopic/histologic features depending on the immunotherapy type. Infliximab-refractory colitis occurs early, is often high grade, and has adverse endoscopic and histopathologic features.
Keywords: CTLA-4 antigen; immunotherapy; inflammation; programmed cell death 1 receptor.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: GL is a consultant advisor for Aduro, Amgen, Bristol-Myers Squibb, Mass-Array, Merck, MSD, Novartis, OncoSec Medical, Pierre Fabre, Roche, Q biotics and Sandoz. MC is a consultant advisor for BMS, MSD, Novartis, Roche, Pierre Fabre, Sanofi, Merck Serono, Eisai, Nektar, Ideaya and Q biotics. AM is a consultant advisor to BMS, MSD, Novartis, Roche and Pierre-Fabre.
Figures

Similar articles
-
Anti-programmed cell death protein 1 tolerance and efficacy after ipilimumab immunotherapy: observational study of 39 patients.Melanoma Res. 2017 Apr;27(2):110-115. doi: 10.1097/CMR.0000000000000313. Melanoma Res. 2017. PMID: 27926587
-
Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma.Ann Oncol. 2018 Jan 1;29(1):250-255. doi: 10.1093/annonc/mdx642. Ann Oncol. 2018. PMID: 29045547 Free PMC article.
-
Management of infliximab refractory immune checkpoint inhibitor gastrointestinal toxicity: a multicenter case series.J Immunother Cancer. 2024 Jan 31;12(1):e008232. doi: 10.1136/jitc-2023-008232. J Immunother Cancer. 2024. PMID: 38296594 Free PMC article.
-
The role of ipilimumab after anti-PD-1 treatment: two case reports and a literature review.Melanoma Res. 2020 Apr;30(2):209-212. doi: 10.1097/CMR.0000000000000632. Melanoma Res. 2020. PMID: 31348136 Review.
-
Ipilimumab-induced toxicities and the gastroenterologist.J Gastroenterol Hepatol. 2015 Apr;30(4):657-66. doi: 10.1111/jgh.12888. J Gastroenterol Hepatol. 2015. PMID: 25641691 Review.
Cited by
-
18 F-FDG PET/CT features of immune-related adverse events and pitfalls following immunotherapy.J Med Imaging Radiat Oncol. 2022 Jun;66(4):483-494. doi: 10.1111/1754-9485.13390. Epub 2022 Feb 22. J Med Imaging Radiat Oncol. 2022. PMID: 35191204 Free PMC article.
-
Clinic, Endoscopic and Histological Features in Patients Treated with ICI Developing GI Toxicity: Some News and Reappraisal from a Mono-Institutional Experience.Diagnostics (Basel). 2022 Mar 11;12(3):685. doi: 10.3390/diagnostics12030685. Diagnostics (Basel). 2022. PMID: 35328239 Free PMC article.
-
Focus on Immune Checkpoint Inhibitors-related Intestinal Inflammation: From Pathogenesis to Therapeutical Approach.Inflamm Bowel Dis. 2024 Jun 3;30(6):1018-1031. doi: 10.1093/ibd/izad229. Inflamm Bowel Dis. 2024. PMID: 37801695 Free PMC article. Review.
-
Oncolytic viruses improve cancer immunotherapy by reprogramming solid tumor microenvironment.Med Oncol. 2023 Dec 8;41(1):8. doi: 10.1007/s12032-023-02233-0. Med Oncol. 2023. PMID: 38062315 Review.
-
Moderate Colitis Not Requiring Intravenous Steroids Is Associated with Improved Survival in Stage IV Melanoma after Anti-CTLA4 Monotherapy, But Not Combination Therapy.Oncologist. 2022 Sep 2;27(9):799-808. doi: 10.1093/oncolo/oyac108. Oncologist. 2022. PMID: 35666292 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
