Activation of Granule Cell Interneurons by Two Divergent Local Circuit Pathways in the Rat Olfactory Bulb
- PMID: 33234611
- PMCID: PMC7726531
- DOI: 10.1523/JNEUROSCI.0989-20.2020
Activation of Granule Cell Interneurons by Two Divergent Local Circuit Pathways in the Rat Olfactory Bulb
Abstract
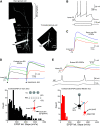
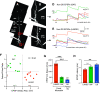
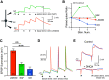
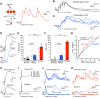
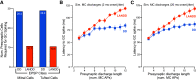
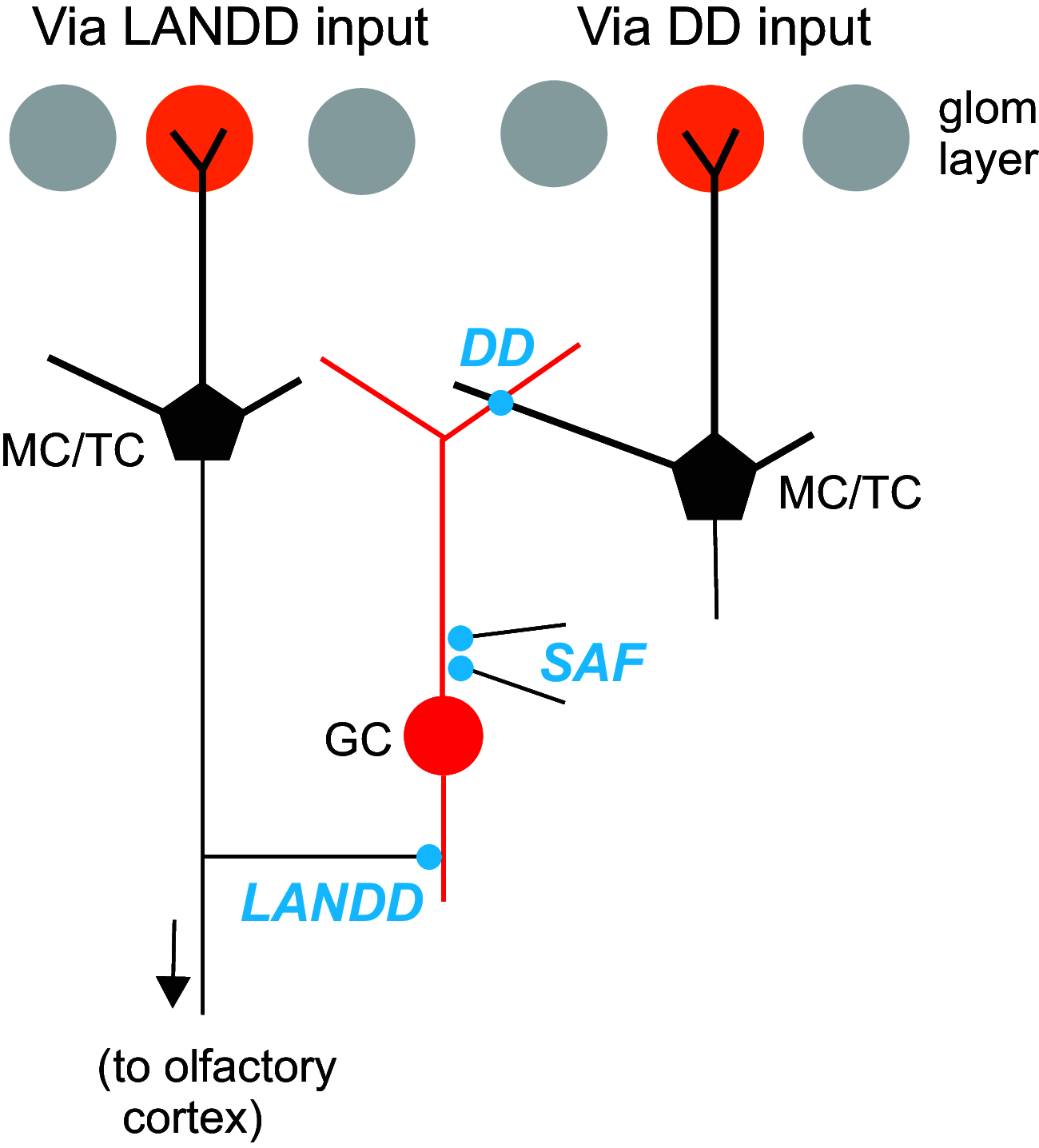
The olfactory bulb (OB) serves as a relay region for sensory information transduced by receptor neurons in the nose and ultimately routed to a variety of cortical areas. Despite the highly structured organization of the sensory inputs to the OB, even simple monomolecular odors activate large regions of the OB comprising many glomerular modules defined by afferents from different receptor neuron subtypes. OB principal cells receive their primary excitatory input from only one glomerular channel defined by inputs from one class of olfactory receptor neurons. By contrast, interneurons, such as GABAergic granule cells (GCs), integrate across multiple channels through dendodendritic inputs on their distal apical dendrites. Through their inhibitory synaptic actions, GCs appear to modulate principal cell firing to enhance olfactory discrimination, although how GCs contribute to olfactory function is not well understood. In this study, we identify a second synaptic pathway by which principal cells in the rat (both sexes) OB excite GCs by evoking potent nondepressing EPSPs (termed large-amplitude, nondendrodendritic [LANDD] EPSPs). LANDD EPSPs show little depression in response to tetanic stimulation and, therefore, can be distinguished other EPSPs that target GCs. LANDD EPSPs can be evoked by both focal stimulation near GC proximal dendrites and by activating sensory inputs in the glomerular layer in truncated GCs lacking dendrodendritic inputs. Using computational simulations, we show that LANDD EPSPs more reliably encode the duration of principal cell discharges than DD EPSPs, enabling GCs to compare contrasting versions of odor-driven activity patterns.SIGNIFICANCE STATEMENT The olfactory bulb plays a critical role in transforming broad sensory input patterns into odor-selective population responses. How this occurs is not well understood, but the local bulbar interneurons appear to be centrally involved in the process. Granule cells, the most common interneuron in the olfactory bulb, are known to broadly integrate sensory input through specialized synapses on their distal dendrites. Here we describe a second class of local excitatory inputs to granule cells that are more powerful than distal inputs and fail to depress with repeated stimulation. This second, proximal pathway allows bulbar interneurons to assay divergent versions of the same sensory input pattern.
Keywords: brain slice; interneurons; olfactory bulb; patch clamp; short-term plasticity.
Copyright © 2020 the authors.
Figures



Similar articles
-
Functional Specialization of Interneuron Dendrites: Identification of Action Potential Initiation Zone in Axonless Olfactory Bulb Granule Cells.J Neurosci. 2019 Dec 4;39(49):9674-9688. doi: 10.1523/JNEUROSCI.1763-19.2019. Epub 2019 Oct 29. J Neurosci. 2019. PMID: 31662426 Free PMC article.
-
Direct Recording of Dendrodendritic Excitation in the Olfactory Bulb: Divergent Properties of Local and External Glutamatergic Inputs Govern Synaptic Integration in Granule Cells.J Neurosci. 2017 Dec 6;37(49):11774-11788. doi: 10.1523/JNEUROSCI.2033-17.2017. Epub 2017 Oct 24. J Neurosci. 2017. PMID: 29066560 Free PMC article.
-
CCKergic Tufted Cells Differentially Drive Two Anatomically Segregated Inhibitory Circuits in the Mouse Olfactory Bulb.J Neurosci. 2020 Aug 5;40(32):6189-6206. doi: 10.1523/JNEUROSCI.0769-20.2020. Epub 2020 Jun 30. J Neurosci. 2020. PMID: 32605937 Free PMC article.
-
Functional organization of the main olfactory bulb.Microsc Res Tech. 1993 Feb 1;24(2):142-56. doi: 10.1002/jemt.1070240206. Microsc Res Tech. 1993. PMID: 8457726 Review.
-
Centrifugal drive onto local inhibitory interneurons of the olfactory bulb.Ann N Y Acad Sci. 2009 Jul;1170:239-54. doi: 10.1111/j.1749-6632.2009.03913.x. Ann N Y Acad Sci. 2009. PMID: 19686142 Review.
Cited by
-
Fast-spiking interneuron detonation drives high-fidelity inhibition in the olfactory bulb.bioRxiv [Preprint]. 2024 May 8:2024.05.07.592874. doi: 10.1101/2024.05.07.592874. bioRxiv. 2024. Update in: PLoS Biol. 2024 Aug 26;22(8):e3002660. doi: 10.1371/journal.pbio.3002660. PMID: 38766161 Free PMC article. Updated. Preprint.
-
Fast-spiking interneuron detonation drives high-fidelity inhibition in the olfactory bulb.PLoS Biol. 2024 Aug 26;22(8):e3002660. doi: 10.1371/journal.pbio.3002660. eCollection 2024 Aug. PLoS Biol. 2024. PMID: 39186804 Free PMC article.
-
Anatomical and Functional Connectivity at the Dendrodendritic Reciprocal Mitral Cell-Granule Cell Synapse: Impact on Recurrent and Lateral Inhibition.Front Neural Circuits. 2022 Jul 22;16:933201. doi: 10.3389/fncir.2022.933201. eCollection 2022. Front Neural Circuits. 2022. PMID: 35937203 Free PMC article.
-
An Evolutionary Microcircuit Approach to the Neural Basis of High Dimensional Sensory Processing in Olfaction.Front Cell Neurosci. 2021 Apr 30;15:658480. doi: 10.3389/fncel.2021.658480. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33994949 Free PMC article.
-
Neuronal Adenosine A1 Receptor is Critical for Olfactory Function but Unable to Attenuate Olfactory Dysfunction in Neuroinflammation.Front Cell Neurosci. 2022 Jun 30;16:912030. doi: 10.3389/fncel.2022.912030. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35846561 Free PMC article.
References
-
- Banerjee A, Dave RN (2004) Validating clusters using the Hopkins statistic. In: IEEE International Conference on Fuzzy Systems, pp 149–153, Vol 1, Budapest, Hungary.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
