Harmine enhances the activity of the HIV-1 latency-reversing agents ingenol A and SAHA
- PMID: 33234703
- PMCID: PMC7774897
- DOI: 10.1242/bio.052969
Harmine enhances the activity of the HIV-1 latency-reversing agents ingenol A and SAHA
Abstract
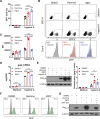
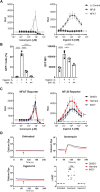
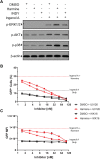
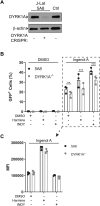
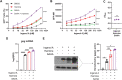
Infection with human immunodeficiency virus 1 (HIV-1) remains incurable because long-lived, latently-infected cells persist during prolonged antiretroviral therapy. Attempts to pharmacologically reactivate and purge the latent reservoir with latency reactivating agents (LRAs) such as protein kinase C (PKC) agonists (e.g. ingenol A) or histone deacetylase (HDAC) inhibitors (e.g. SAHA) have shown promising but incomplete efficacy. Using the J-Lat T cell model of HIV latency, we found that the plant-derived compound harmine enhanced the efficacy of existing PKC agonist LRAs in reactivating latently-infected cells. Treatment with harmine increased not only the number of reactivated cells but also increased HIV transcription and protein expression on a per-cell basis. Importantly, we observed a synergistic effect when harmine was used in combination with ingenol A and the HDAC inhibitor SAHA. An investigation into the mechanism revealed that harmine, when used with LRAs, increased the activity of NFκB, MAPK p38, and ERK1/2. Harmine treatment also resulted in reduced expression of HEXIM1, a negative regulator of transcriptional elongation. Thus, harmine enhanced the effects of LRAs by increasing the availability of transcription factors needed for HIV reactivation and promoting transcriptional elongation. Combination therapies with harmine and LRAs could benefit patients by achieving deeper reactivation of the latent pool of HIV provirus.
Keywords: HIV; Harmine; Latency.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Archin N. M., Liberty A. L., Kashuba A. D., Choudhary S. K., Kuruc J. D., Crooks A. M., Parker D. C., Anderson E. M., Kearney M. F., Strain M. C. et al. (2012). Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487, 482-485. 10.1038/nature11286 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

