Biomineral armor in leaf-cutter ants
- PMID: 33235196
- PMCID: PMC7686325
- DOI: 10.1038/s41467-020-19566-3
Biomineral armor in leaf-cutter ants
Abstract
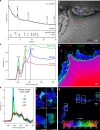
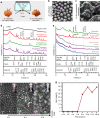
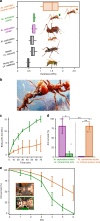
Although calcareous anatomical structures have evolved in diverse animal groups, such structures have been unknown in insects. Here, we report the discovery of high-magnesium calcite [CaMg(CO3)2] armor overlaying the exoskeletons of major workers of the leaf-cutter ant Acromyrmex echinatior. Live-rearing and in vitro synthesis experiments indicate that the biomineral layer accumulates rapidly as ant workers mature, that the layer is continuously distributed, covering nearly the entire integument, and that the ant epicuticle catalyzes biomineral nucleation and growth. In situ nanoindentation demonstrates that the biomineral layer significantly hardens the exoskeleton. Increased survival of ant workers with biomineralized exoskeletons during aggressive encounters with other ants and reduced infection by entomopathogenic fungi demonstrate the protective role of the biomineral layer. The discovery of biogenic high-magnesium calcite in the relatively well-studied leaf-cutting ants suggests that calcareous biominerals enriched in magnesium may be more common in metazoans than previously recognized.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Knoll AH. Biomineralization and evolutionary history. Rev. Mineral. Geochem. 2003;54:329–356. doi: 10.2113/0540329. - DOI
-
- Gilbert PUPA, Abrecht M, Frazer BH. The organic-mineral interface in biominerals. Mol. Geomicrobiol. 2018;59:157–185.

