Addressing the challenges of E-cigarette safety profiling by assessment of pulmonary toxicological response in bronchial and alveolar mucosa models
- PMID: 33235237
- PMCID: PMC7686373
- DOI: 10.1038/s41598-020-77452-w
Addressing the challenges of E-cigarette safety profiling by assessment of pulmonary toxicological response in bronchial and alveolar mucosa models
Abstract
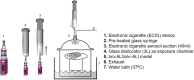
Limited toxicity data on electronic cigarette (ECIG) impede evidence-based policy recommendations. We compared two popular mixed fruit flavored ECIG-liquids with and without nicotine aerosolized at 40 W (E-smoke) with respect to particle number concentrations, chemical composition, and response on physiologically relevant human bronchial and alveolar lung mucosa models cultured at air-liquid interface. E-smoke was characterized by significantly increased particle number concentrations with increased wattage (25, 40, and 55 W) and nicotine presence. The chemical composition of E-smoke differed across the two tested flavors in terms of cytotoxic compounds including p-benzoquinone, nicotyrine, and flavoring agents (for example vanillin, ethyl vanillin). Significant differences in the expression of markers for pro-inflammation, oxidative stress, tissue injury/repair, alarm anti-protease, anti-microbial defense, epithelial barrier function, and epigenetic modification were observed between the flavors, nicotine content, and/ or lung models (bronchial or alveolar). Our findings indicate that ECIG toxicity is influenced by combination of multiple factors including flavor, nicotine content, vaping regime, and the region of respiratory tree (bronchial or alveolar). Toxic chemicals and flavoring agents detected in high concentrations in the E-smoke of each flavor warrant independent evaluation for their specific role in imparting toxicity. Therefore, multi-disciplinary approaches are warranted for comprehensive safety profiling of ECIG.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Sub-ohm vaping increases the levels of carbonyls, is cytotoxic, and alters gene expression in human bronchial epithelial cells exposed at the air-liquid interface.Respir Res. 2020 Nov 19;21(1):305. doi: 10.1186/s12931-020-01571-1. Respir Res. 2020. PMID: 33213456 Free PMC article.
-
Chemical Adducts of Reactive Flavor Aldehydes Formed in E-Cigarette Liquids Are Cytotoxic and Inhibit Mitochondrial Function in Respiratory Epithelial Cells.Nicotine Tob Res. 2020 Dec 15;22(Suppl 1):S25-S34. doi: 10.1093/ntr/ntaa185. Nicotine Tob Res. 2020. PMID: 33320255 Free PMC article.
-
Variations in coil temperature/power and e-liquid constituents change size and lung deposition of particles emitted by an electronic cigarette.Physiol Rep. 2019 May;7(10):e14093. doi: 10.14814/phy2.14093. Physiol Rep. 2019. PMID: 31140749 Free PMC article.
-
Vaping versus Smoking: A Quest for Efficacy and Safety of E-cigarette.Curr Drug Saf. 2018;13(2):92-101. doi: 10.2174/1574886313666180227110556. Curr Drug Saf. 2018. PMID: 29485005 Review.
-
A Summary of In Vitro and In Vivo Studies Evaluating the Impact of E-Cigarette Exposure on Living Organisms and the Environment.Int J Mol Sci. 2020 Jan 19;21(2):652. doi: 10.3390/ijms21020652. Int J Mol Sci. 2020. PMID: 31963832 Free PMC article. Review.
Cited by
-
Pulmonary effects of e-liquid flavors: a systematic review.J Toxicol Environ Health B Crit Rev. 2022 Oct 3;25(7):343-371. doi: 10.1080/10937404.2022.2124563. Epub 2022 Sep 25. J Toxicol Environ Health B Crit Rev. 2022. PMID: 36154615 Free PMC article.
-
Acute and chronic effects of vaping electronic devices on lung physiology and inflammation.Curr Opin Physiol. 2021 Aug;22:100447. doi: 10.1016/j.cophys.2021.06.001. Epub 2021 Jun 11. Curr Opin Physiol. 2021. PMID: 38550798 Free PMC article.
-
Airway-On-A-Chip: Designs and Applications for Lung Repair and Disease.Cells. 2021 Jun 26;10(7):1602. doi: 10.3390/cells10071602. Cells. 2021. PMID: 34206722 Free PMC article. Review.
-
Circulating Secretoglobin Family 1A Member 1 (SCGB1A1) Levels as a Marker of Biomass Smoke Induced Chronic Obstructive Pulmonary Disease.Toxics. 2021 Aug 31;9(9):208. doi: 10.3390/toxics9090208. Toxics. 2021. PMID: 34564359 Free PMC article.
-
Local and systemic effects in e-cigarette users compared to cigarette smokers, dual users, and non-smokers.Respir Res. 2025 Jun 4;26(1):207. doi: 10.1186/s12931-025-03289-4. Respir Res. 2025. PMID: 40468357 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

