Immortalisation of primary human alveolar epithelial lung cells using a non-viral vector to study respiratory bioreactivity in vitro
- PMID: 33235275
- PMCID: PMC7686381
- DOI: 10.1038/s41598-020-77191-y
Immortalisation of primary human alveolar epithelial lung cells using a non-viral vector to study respiratory bioreactivity in vitro
Abstract
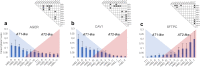
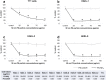
To overcome the scarcity of primary human alveolar epithelial cells for lung research, and the limitations of current cell lines to recapitulate the phenotype, functional and molecular characteristics of the healthy human alveolar epithelium, we have developed a new method to immortalise primary human alveolar epithelial lung cells using a non-viral vector to transfect the telomerase catalytic subunit (hTERT) and the simian virus 40 large-tumour antigen (SV40). Twelve strains of immortalised cells (ICs) were generated and characterised using molecular, immunochemical and morphological techniques. Cell proliferation and sensitivity to polystyrene nanoparticles (PS) were evaluated. ICs expressed caveolin-1, podoplanin and receptor for advanced glycation end-products (RAGE), and most cells were negative for alkaline phosphatase staining, indicating characteristics of AT1-like cells. However, most strains also contained some cells that expressed pro-surfactant protein C, classically described to be expressed only by AT2 cells. Thus, the ICs mimic the cellular heterogeneity in the human alveolar epithelium. These ICs can be passaged, replicate rapidly and remain confluent beyond 15 days. ICs showed differential sensitivity to positive and negatively charged PS nanoparticles, illustrating their potential value as an in vitro model to study respiratory bioreactivity. These novel ICs offer a unique resource to study human alveolar epithelial biology.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Immortalization of human alveolar epithelial cells to investigate nanoparticle uptake.Am J Respir Cell Mol Biol. 2008 Nov;39(5):591-7. doi: 10.1165/rcmb.2007-0334OC. Epub 2008 Jun 6. Am J Respir Cell Mol Biol. 2008. PMID: 18539954 Free PMC article.
-
Differential bioreactivity of neutral, cationic and anionic polystyrene nanoparticles with cells from the human alveolar compartment: robust response of alveolar type 1 epithelial cells.Part Fibre Toxicol. 2015 Jul 2;12:19. doi: 10.1186/s12989-015-0091-7. Part Fibre Toxicol. 2015. PMID: 26133975 Free PMC article.
-
Efficient Transcriptionally Controlled Plasmid Expression System for Investigation of the Stability of mRNA Transcripts in Primary Alveolar Epithelial Cells.J Vis Exp. 2020 Mar 6;(157). doi: 10.3791/60654. J Vis Exp. 2020. PMID: 32202533
-
On Top of the Alveolar Epithelium: Surfactant and the Glycocalyx.Int J Mol Sci. 2020 Apr 27;21(9):3075. doi: 10.3390/ijms21093075. Int J Mol Sci. 2020. PMID: 32349261 Free PMC article. Review.
-
Function of epithelial stem cell in the repair of alveolar injury.Stem Cell Res Ther. 2022 Apr 27;13(1):170. doi: 10.1186/s13287-022-02847-7. Stem Cell Res Ther. 2022. PMID: 35477551 Free PMC article. Review.
Cited by
-
Human Brain In Vitro Model for Pathogen Infection-Related Neurodegeneration Study.Int J Mol Sci. 2024 Jun 13;25(12):6522. doi: 10.3390/ijms25126522. Int J Mol Sci. 2024. PMID: 38928228 Free PMC article. Review.
-
Evidence of Mpox clade IIb infection in primary human alveolar epithelium.Emerg Microbes Infect. 2025 Dec;14(1):2477845. doi: 10.1080/22221751.2025.2477845. Epub 2025 Mar 25. Emerg Microbes Infect. 2025. PMID: 40059759 Free PMC article.
-
Emerging Technologies for In Vitro Inhalation Toxicology.Adv Healthc Mater. 2021 Sep;10(18):e2100633. doi: 10.1002/adhm.202100633. Epub 2021 Jul 22. Adv Healthc Mater. 2021. PMID: 34292676 Free PMC article. Review.
-
Bioinformatics and Experimental Study Revealed LINC00982/ miR-183-5p/ABCA8 Axis Suppresses LUAD Progression.Curr Cancer Drug Targets. 2024;24(6):654-667. doi: 10.2174/0115680096266700231107071222. Curr Cancer Drug Targets. 2024. PMID: 38419344
-
Gene Therapeutics for Surfactant Dysfunction Disorders: Targeting the Alveolar Type 2 Epithelial Cell.Hum Gene Ther. 2022 Oct;33(19-20):1011-1022. doi: 10.1089/hum.2022.130. Hum Gene Ther. 2022. PMID: 36166236 Free PMC article. Review.
References
-
- Witherden IR, Tetley TD. Isolation and culture of human type II pneumocytes. In: Rogers DF, Donnelly LE, editors. Human Airway Inflammation: Sampling Techniques and Analytical Protocols. Totowa: Human Press Inc; 2001. pp. 137–146.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

