Efficacy of infliximab in the treatment of Kawasaki disease: A systematic review and meta-analysis
- PMID: 33235624
- PMCID: PMC7678622
- DOI: 10.3892/etm.2020.9447
Efficacy of infliximab in the treatment of Kawasaki disease: A systematic review and meta-analysis
Abstract
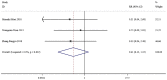
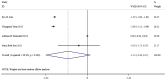
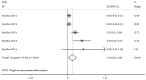
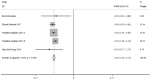
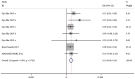
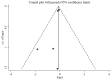
The present study aimed to review the relevant studies in order to determine the efficacy of infliximab (IFX) in the treatment of Kawasaki disease (KD). The relevant studies were retrieved using the PubMed, Cochrane and Embase databases. Key sources in the literature were reviewed; all articles published by July 2019 were considered for inclusion. For each study, odds ratios, mean difference and 95% confidence interval (95% CI) were assessed to evaluate study outcomes. A total of 16 studies involving 429 patients were relevant to the questions of interest of the current meta-analysis. Compared with intravenous immunoglobulin (IVIG), IFX or IFX plus IVIG significantly reduced the incidence of adverse events, including the number of patients with fever, changes in lip and oral cavity and/or cervical lymphadenopathy. The white blood cell (WBC), neutrophil and C-reactive protein (CRP) levels were also reduced in the IFX or IFX plus IVIG group compared with those in the IVIG or polyethylene glycol-treated human immunoglobulin (VGIH) groups. The platelet counts, alanine aminotransferase (ALT) levels and Z-scores were increased in the IFX or IFX plus IVIG groups compared with those in the IVIG or VGIH groups. In the single-arm studies, the incidence of coronary artery aneurysm was 0.150 (95% CI: 0.024, 0.277), the non-response rate was 0.097 (95% CI: 0.056, 0.138), and the incidence of adverse events was 0.156 (95% CI: 0.122, 0.190). IFX not only effectively reduced the incidence of fever, conjunctival injection, changes in lip and oral cavity and cervical lymphadenopathy polymorphous exanthema, but also the WBC, neutrophil, ALT and CRP levels. The platelet levels were increased in patients after the IFX therapy compared with patients in the IVIG or VGIH groups. IFX or IFX plus IVIG exhibited improved clinical efficacy in the treatment of KD compared with that of IVIG or VGIH. However, as a limited number of studies was included in the current study, the findings should be verified further.
Keywords: Kawasaki disease; infliximab; meta-analysis.
Copyright: © Lu et al.
Figures



Similar articles
-
The effectiveness of infliximab for Kawasaki disease in children: systematic review and meta-analysis.Transl Pediatr. 2021 May;10(5):1294-1306. doi: 10.21037/tp-20-482. Transl Pediatr. 2021. PMID: 34189087 Free PMC article.
-
Comparison of second-line therapy in IVIg-refractory Kawasaki disease: a systematic review.Pediatr Rheumatol Online J. 2019 Nov 27;17(1):77. doi: 10.1186/s12969-019-0380-z. Pediatr Rheumatol Online J. 2019. PMID: 31775898 Free PMC article.
-
Predictors of intravenous immunoglobulin-resistant Kawasaki disease in children: a meta-analysis of 4442 cases.Eur J Pediatr. 2018 Aug;177(8):1279-1292. doi: 10.1007/s00431-018-3182-2. Epub 2018 Jun 8. Eur J Pediatr. 2018. PMID: 29948255 Free PMC article. Review.
-
Infliximab versus intravenous immunoglobulin for refractory Kawasaki disease: a phase 3, randomized, open-label, active-controlled, parallel-group, multicenter trial.Sci Rep. 2018 Jan 31;8(1):1994. doi: 10.1038/s41598-017-18387-7. Sci Rep. 2018. PMID: 29386515 Free PMC article. Clinical Trial.
-
Intravenous Immunoglobulin Gamma (IVIG) versus IVIG Plus Infliximab in Young Children with Kawasaki Disease.Med Sci Monit. 2018 Oct 11;24:7264-7270. doi: 10.12659/MSM.908678. Med Sci Monit. 2018. PMID: 30307902 Free PMC article. Clinical Trial.
Cited by
-
Assessing the efficacy of infliximab in promoting vascular and mucosal healing in immunoglobulin-resistant kawasaki disease: a meta-analysis.BMC Pediatr. 2025 Feb 27;25(1):147. doi: 10.1186/s12887-025-05482-2. BMC Pediatr. 2025. PMID: 40011861 Free PMC article.
-
The Network of Pro-Inflammatory Factors CD147, DcR3, and IL33 in the Development of Kawasaki Disease.J Inflamm Res. 2021 Nov 19;14:6043-6053. doi: 10.2147/JIR.S338763. eCollection 2021. J Inflamm Res. 2021. PMID: 34824540 Free PMC article.
References
-
- Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. (In Japanese) - PubMed
-
- Soriano-Ramos M, Martínez-Del Val E, Negreira Cepeda S, González-Tomé MI, Cedena Romero P, Fernández-Cooke E, Albert de la Torre L, Blázquez-Gamero D. Risk of coronary artery involvement in Kawasaki disease. Arch Argent Pediatr. 2016;114:107–113. doi: 10.5546/aap.2016.eng.107. (In English, Spanish) - DOI - PubMed
-
- Lin Y, Xiao-hui L and Shi L: Interpretation of the 2017 edition of diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American heart association. Chin J Pract Pediatr, 2017 (In Chinese).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
