This is a preprint.
Patterns and persistence of SARS-CoV-2 IgG antibodies in Chicago to monitor COVID-19 exposure
- PMID: 33236031
- PMCID: PMC7685344
- DOI: 10.1101/2020.11.17.20233452
Patterns and persistence of SARS-CoV-2 IgG antibodies in Chicago to monitor COVID-19 exposure
Update in
-
Patterns and persistence of SARS-CoV-2 IgG antibodies in Chicago to monitor COVID-19 exposure.JCI Insight. 2021 May 10;6(9):e146148. doi: 10.1172/jci.insight.146148. JCI Insight. 2021. PMID: 33755598 Free PMC article.
Abstract
Background: Estimates of seroprevalence to SARS-CoV-2 vary widely and may influence vaccination response. We ascertained IgG levels across a single US metropolitan site, Chicago, from June 2020 through December 2020.
Methods: Participants (n=7935) were recruited through electronic advertising and received materials for a self-sampled dried blood spot assay through the mail or a minimal contact in person method. IgG to the receptor binding domain of SARS-CoV-2 was measured using an established highly sensitive and highly specific assay.
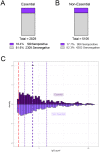
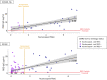
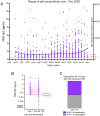
Results: Overall seroprevalence was 17.9%, with no significant difference between method of contact. Only 2.5% of participants reported having had a diagnosis of COVID-19 based on virus detection, consistent with a 7-fold greater exposure to SARS-CoV-2 measured by serology than detected by viral testing. The range of IgG level observed in seropositive participants from this community survey overlapped with the range of IgG levels associated with COVID-19 cases having a documented positive PCR positive test. From a subset of those who participated in repeat testing, half of seropositive individuals retained detectable antibodies for 3-4 months.
Conclusions: Quantitative IgG measurements with a highly specific and sensitive assay indicate more widespread exposure to SARS-CoV-2 than observed by viral testing. The range of IgG concentration produced from these asymptomatic exposures is similar to IgG levels occurring after documented non-hospitalized COVID-19, which is considerably lower than that produced from hospitalized COVID-19 cases. The differing ranges of IgG response, coupled with the rate of decay of antibodies, may influence response to subsequent viral exposure and vaccine.
Keywords: COVID-19; ELISA; IgG; SARS-CoV-2; dried blood spots; essential worker; nucleocapsid; receptor binding domain; serological testing.
Conflict of interest statement
Conflicts of Interest: Thomas McDade has a financial interest in EnMed Microanalytics, a company that specializes in laboratory testing of dried blood spot samples. All other authors declare no conflicts of interest.
Figures

Similar articles
-
Patterns and persistence of SARS-CoV-2 IgG antibodies in Chicago to monitor COVID-19 exposure.JCI Insight. 2021 May 10;6(9):e146148. doi: 10.1172/jci.insight.146148. JCI Insight. 2021. PMID: 33755598 Free PMC article.
-
High seroprevalence for SARS-CoV-2 among household members of essential workers detected using a dried blood spot assay.PLoS One. 2020 Aug 14;15(8):e0237833. doi: 10.1371/journal.pone.0237833. eCollection 2020. PLoS One. 2020. PMID: 32797108 Free PMC article.
-
A Saliva-Based Serological and Behavioral Analysis of SARS-CoV-2 Antibody Prevalence in Howard County, Maryland.Microbiol Spectr. 2023 Aug 17;11(4):e0276522. doi: 10.1128/spectrum.02765-22. Epub 2023 Jun 8. Microbiol Spectr. 2023. PMID: 37289070 Free PMC article.
-
Utility of Newborn Dried Blood Spots to Ascertain Seroprevalence of SARS-CoV-2 Antibodies Among Individuals Giving Birth in New York State, November 2019 to November 2021.JAMA Netw Open. 2022 Aug 1;5(8):e2227995. doi: 10.1001/jamanetworkopen.2022.27995. JAMA Netw Open. 2022. PMID: 35994287 Free PMC article.
-
SARS-CoV-2 Antibody Testing in Health Care Workers: A Comparison of the Clinical Performance of Three Commercially Available Antibody Assays.Microbiol Spectr. 2021 Oct 31;9(2):e0039121. doi: 10.1128/Spectrum.00391-21. Epub 2021 Sep 29. Microbiol Spectr. 2021. PMID: 34585976 Free PMC article.
References
-
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, Adriano A, Beese S, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Dittrich S, Emperador D, Hooft L, Leeflang MM, and Van den Bruel A. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6(6):Cd013652. - PMC - PubMed
-
- Figueiredo-Campos P, Blankenhaus B, Mota C, Gomes A, Serrano M, Ariotti S, Costa C, Nunes-Cabaço H, Mendes AM, Gaspar P, Pereira-Santos MC, Rodrigues F, Condeço J, Escoval MA, Santos M, Ramirez M, Melo-Cristino J, Simas JP, Vasconcelos E, Afonso Â, and Veldhoen M. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to six months post disease onset. Eur J Immunol. 2020. - PMC - PubMed
-
- Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E, Arnthorsson AO, Helgason D, Bjarnadottir K, Ingvarsson RF, Thorsteinsdottir B, Kristjansdottir S, Birgisdottir K, Kristinsdottir AM, Sigurdsson MI, Arnadottir GA, Ivarsdottir EV, Andresdottir M, Jonsson F, Agustsdottir AB, Berglund J, Eiriksdottir B, Fridriksdottir R, Gardarsdottir EE, Gottfredsson M, Gretarsdottir OS, Gudmundsdottir S, Gudmundsson KR, Gunnarsdottir TR, Gylfason A, Helgason A, Jensson BO, Jonasdottir A, Jonsson H, Kristjansson T, Kristinsson KG, Magnusdottir DN, Magnusson OT, Olafsdottir LB, Rognvaldsson S, le Roux L, Sigmundsdottir G, Sigurdsson A, Sveinbjornsson G, Sveinsdottir KE, Sveinsdottir M, Thorarensen EA, Thorbjornsson B, Thordardottir M, Saemundsdottir J, Kristjansson SH, Josefsdottir KS, Masson G, Georgsson G, Kristjansson M, Moller A, Palsson R, Gudnason T, Thorsteinsdottir U, Jonsdottir I, Sulem P, and Stefansson K. Humoral Immune Response to SARS-CoV-2 in Iceland. N Engl J Med. 2020. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
