This is a preprint.
Estimating the cumulative incidence of SARS-CoV-2 infection and the infection fatality ratio in light of waning antibodies
- PMID: 33236035
- PMCID: PMC7685353
- DOI: 10.1101/2020.11.13.20231266
Estimating the cumulative incidence of SARS-CoV-2 infection and the infection fatality ratio in light of waning antibodies
Update in
-
Estimating the Cumulative Incidence of SARS-CoV-2 Infection and the Infection Fatality Ratio in Light of Waning Antibodies.Epidemiology. 2021 Jul 1;32(4):518-524. doi: 10.1097/EDE.0000000000001361. Epidemiology. 2021. PMID: 33935138 Free PMC article.
Abstract
Background: Serology tests can identify previous infections and facilitate estimation of the number of total infections. However, immunoglobulins targeting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been reported to wane below the detectable level of serological assays. We estimate the cumulative incidence of SARS-CoV-2 infection from serology studies, accounting for expected levels of antibody acquisition (seroconversion) and waning (seroreversion), and apply this framework using data from New York City (NYC) and Connecticut.
Methods: We estimated time from seroconversion to seroreversion and infection fatality ratio (IFR) using mortality data from March-October 2020 and population-level cross-sectional seroprevalence data from April-August 2020 in NYC and Connecticut. We then estimated the daily seroprevalence and cumulative incidence of SARS-CoV-2 infection.
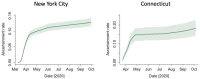
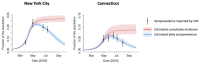
Findings: The estimated average time from seroconversion to seroreversion was 3-4 months. The estimated IFR was 1.1% (95% credible interval: 1.0-1.2%) in NYC and 1.4% (1.1-1.7%) in Connecticut. The estimated daily seroprevalence declined after a peak in the spring. The estimated cumulative incidence reached 26.8% (24.2-29.7%) and 8.8% (7.1-11.3%) at the end of September in NYC and Connecticut, higher than maximum seroprevalence measures (22.1% and 6.1%), respectively.
Interpretation: The cumulative incidence of SARS-CoV-2 infection is underestimated using cross-sectional serology data without adjustment for waning antibodies. Our approach can help quantify the magnitude of underestimation and adjust estimates for waning antibodies.
Funding: This study was supported by the US National Science Foundation and the National Institute of Allergy and Infectious Diseases.
Keywords: Antibody; COVID-19; SARS-CoV-2; ascertainment bias; case ascertainment ratio; cumulative incidence; infection fatality ratio; seroconversion; seroprevalence; seroreversion; waning antibody.
Conflict of interest statement
Conflicts of interest BAL reports personal fees from Takeda Pharmaceutical, personal fees from CDC Foundation, and personal fees from Hall Booth Smith, P.C., outside the submitted work. Other authors do not have conflicts of interest.
Figures


References
-
- COVID-19 Map - Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html (accessed Oct 8, 2020).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
