Practical challenges to the clinical implementation of saliva for SARS-CoV-2 detection
- PMID: 33236269
- PMCID: PMC7685775
- DOI: 10.1007/s10096-020-04090-5
Practical challenges to the clinical implementation of saliva for SARS-CoV-2 detection
Abstract
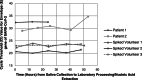
Due to global shortages of flocked nasopharyngeal swabs and appropriate viral transport media during the COVID-19 pandemic, alternate diagnostic specimens for SARS-CoV-2 detection are sought. The accuracy and feasibility of saliva samples collected and transported without specialized collection devices or media were evaluated. Saliva demonstrated good concordance with paired nasopharyngeal swabs for SARS-CoV-2 detection in 67/74 cases (90.5%), though barriers to saliva collection were observed in long-term care residents and outbreak settings. SARS-CoV-2 RNA was stable in human saliva at room temperature for up to 48 h after initial specimen collection, informing appropriate transport time and conditions.
Keywords: COVID-19; Nasopharyngeal; SARS-CoV-2; Saliva; Transport.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Comparison of SARS-CoV-2 PCR-Based Detection Using Saliva or Nasopharyngeal Swab Specimens in Asymptomatic Populations.Microbiol Spectr. 2021 Sep 3;9(1):e0006221. doi: 10.1128/Spectrum.00062-21. Epub 2021 Aug 25. Microbiol Spectr. 2021. PMID: 34431689 Free PMC article.
-
Detection and Stability of SARS-CoV-2 in Three Self-Collected Specimen Types: Flocked Midturbinate Swab (MTS) in Viral Transport Media, Foam MTS, and Saliva.Microbiol Spectr. 2022 Jun 29;10(3):e0103322. doi: 10.1128/spectrum.01033-22. Epub 2022 Jun 6. Microbiol Spectr. 2022. PMID: 35665629 Free PMC article.
-
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Sep;21(9):1233-1245. doi: 10.1016/S1473-3099(21)00146-8. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857405 Free PMC article.
-
Relative Sensitivity of Saliva and Upper Airway Swabs for Initial Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Ambulatory Patients: Rapid Review.J Mol Diagn. 2021 Mar;23(3):265-273. doi: 10.1016/j.jmoldx.2020.12.008. Epub 2021 Jan 1. J Mol Diagn. 2021. PMID: 33395576 Free PMC article. Review.
Cited by
-
Saliva as an alternative specimen to nasopharyngeal swabs for COVID-19 diagnosis: Review.Access Microbiol. 2022 May 20;4(5):acmi000366. doi: 10.1099/acmi.0.000366. eCollection 2022 Aug. Access Microbiol. 2022. PMID: 36003360 Free PMC article. Review.
-
Saliva as a gold-standard sample for SARS-CoV-2 detection.Lancet Respir Med. 2021 Jun;9(6):562-564. doi: 10.1016/S2213-2600(21)00178-8. Epub 2021 Apr 19. Lancet Respir Med. 2021. PMID: 33887248 Free PMC article. No abstract available.
-
Self-sampling to identify pathogens and inflammatory markers in patients with acute sore throat: Feasibility study.Front Immunol. 2022 Oct 6;13:1016181. doi: 10.3389/fimmu.2022.1016181. eCollection 2022. Front Immunol. 2022. PMID: 36275691 Free PMC article.
-
Non-invasive SARS-CoV-2 RNA detection and human transcriptome analysis using skin surface lipids.Sci Rep. 2024 Oct 30;14(1):26057. doi: 10.1038/s41598-024-77862-0. Sci Rep. 2024. PMID: 39472469 Free PMC article.
-
The reliability of saliva for the detection of SARS-CoV-2 in symptomatic and asymptomatic patients: Insights on the diagnostic performance and utility for COVID-19 screening.Diagn Microbiol Infect Dis. 2021 Nov;101(3):115450. doi: 10.1016/j.diagmicrobio.2021.115450. Epub 2021 Jun 6. Diagn Microbiol Infect Dis. 2021. PMID: 34284319 Free PMC article.
References
-
- World Health Organization (2020) 2019 Novel Coronavirus (2019-nCoV): strategic preparedness and response plan. 28. Available at: https://www.who.int/publications-detail/strategic-preparedness-and-respo.... Accessed 4 May 2020
-
- Baron EJ, Miller JM, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the infectious diseases society of America (IDSA) and the American Society for Microbiology (ASM) Clin Infect Dis. 2013;57:e22–121. doi: 10.1093/cid/cit278. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

