Crosstalk between the activated Slit2-Robo1 pathway and TGF-β1 signalling promotes cardiac fibrosis
- PMID: 33236535
- PMCID: PMC7835586
- DOI: 10.1002/ehf2.13095
Crosstalk between the activated Slit2-Robo1 pathway and TGF-β1 signalling promotes cardiac fibrosis
Abstract
Aims: Previous reports indicated that the Slit2-Robo signalling pathway is involved in embryonic heart development and fibrosis in other solid organs, but its function in adult cardiac fibrosis has not been investigated. Here, we investigate the role of the Slit2-Robo1 signalling pathway in cardiac fibrosis.
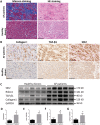
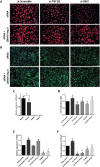
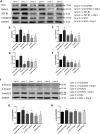
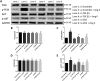
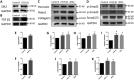
Methods and results: The right atrial tissue samples were obtained from patients with valvular heart disease complicated by atrial fibrillation during heart valve surgery and from healthy heart donors. The fibrotic animal model is created by performing transverse aortic constriction (TAC) surgery. The Robo1, Slit2, TGF-β1, and collagen I expression levels in human and animal samples were evaluated by immunohistochemistry and western blot analysis. Echocardiography measured the changes in heart size and cardiac functions of animals. Angiotensin II (Ang II), Slit2-siRNA, TGF-β1-siRNA, recombinant Slit2, and recombinant TGF-β1 were transfected to cardiac fibroblasts (CFs) respectively to observe their effects on collagen I expression level. The right atrial appendage of patients with valvular heart disease complicated by atrial fibrillation found significantly up-regulated Slit2, Robo1, TGF-β1, and collagen I expression levels. TAC surgery leads to heart enlargement, cardiac fibrosis, and up-regulation of Slit2, Robo1, TGF-β1, and collagen I expression levels in animal model. Robo1 antagonist R5 and TGF-β1 antagonist SB431542 suppressed cardiac fibrosis in TAC mice. Treatment with 100 nM Ang II in CFs caused significantly increased Slit2, Robo1, Smad2/3, TGF-β1, collagen I, PI3K, and Akt expression levels. Transfecting Slit2-siRNA and TGF-β1-siRNA, respectively, into rat CFs significantly down-regulated Smad2/3 and collagen I expression, inhibiting the effects of Ang II. Recombinant Slit2 activated the TGF-β1/Smad signalling pathway in CFs and up-regulated Periostin, Robo1, and collagen I expression.
Conclusions: The Slit2-Robo1 signalling pathway interfered with the TGF-β1/Smad pathway and promoted cardiac fibrosis. Blockade of Slit2-Robo1 might be a new treatment for cardiac fibrosis.
Keywords: Cardiac fibrosis; Crosstalk; Signalling pathway; Slit2; TGF-β1.
© 2020 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Meagher P, Adam M, Connelly K. It's not all about the cardiomyocyte: fibroblasts, empagliflozin, and cardiac remodelling. Can J Cardiol 2020; 36: 464–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
