Sarcopenia in chronic liver disease: mechanisms and countermeasures
- PMID: 33236953
- PMCID: PMC8609568
- DOI: 10.1152/ajpgi.00373.2020
Sarcopenia in chronic liver disease: mechanisms and countermeasures
Abstract
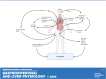
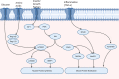
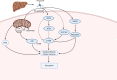
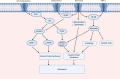
Sarcopenia, a condition of low muscle mass, quality, and strength, is commonly found in patients with cirrhosis and is associated with adverse clinical outcomes including reduction in quality of life, increased mortality, and posttransplant complications. In chronic liver disease (CLD), sarcopenia is most commonly defined through the measurement of the skeletal muscle index of the third lumbar spine. A major contributor to sarcopenia in CLD is the imbalance in muscle protein turnover, which likely occurs due to a decrease in muscle protein synthesis and an elevation in muscle protein breakdown. This imbalance is assumed to arise due to several factors including accelerated starvation, hyperammonemia, amino acid deprivation, chronic inflammation, excessive alcohol intake, and physical inactivity. In particular, hyperammonemia is a key mediator of the liver-gut axis and is known to contribute to mitochondrial dysfunction and an increase in myostatin expression. Currently, the use of nutritional interventions such as late-evening snacks, branched-chain amino acid supplementation, and physical activity have been proposed to help the management and treatment of sarcopenia. However, little evidence exists to comprehensively support their use in clinical settings. Several new pharmacological strategies, including myostatin inhibition and the nutraceutical Urolithin A, have recently been proposed to treat age-related sarcopenia and may also be of use in CLD. This review highlights the potential molecular mechanisms contributing to sarcopenia in CLD alongside a discussion of existing and potential new treatment strategies.
Keywords: chronic liver disease; exercise; muscle protein synthesis; nutrition; sarcopenia.
Conflict of interest statement
The views expressed are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care. No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
-
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48: 16–31, 2019. doi: 10.1093/ageing/afy169. - DOI - PMC - PubMed
-
- Rosenberg IH. Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr 50: 1231–1233, 1989. doi: 10.1093/ajcn/50.5.1231. - DOI
-
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel J-P, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39: 412–423, 2010. doi: 10.1093/ageing/afq034. - DOI - PMC - PubMed
-
- Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr 27: 793–799, 2008. doi: 10.1016/j.clnu.2008.06.013. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

