High-resolution Structural Magnetic Resonance Imaging and Quantitative Susceptibility Mapping
- PMID: 33237013
- PMCID: PMC7886008
- DOI: 10.1016/j.mric.2020.09.002
High-resolution Structural Magnetic Resonance Imaging and Quantitative Susceptibility Mapping
Abstract
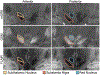
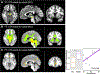
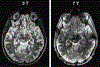
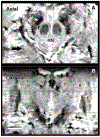
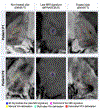
High-resolution 7-T imaging and quantitative susceptibility mapping produce greater anatomic detail compared with conventional strengths because of improvements in signal/noise ratio and contrast. The exquisite anatomic details of deep structures, including delineation of microscopic architecture using advanced techniques such as quantitative susceptibility mapping, allows improved detection of abnormal findings thought to be imperceptible on clinical strengths. This article reviews caveats and techniques for translating sequences commonly used on 1.5 or 3 T to high-resolution 7-T imaging. It discusses for several broad disease categories how high-resolution 7-T imaging can advance the understanding of various diseases, improve diagnosis, and guide management.
Keywords: High-resolution 7 T; Hippocampus; Midbrain; Motion correction; Neurodegenerative diseases; Neuropsychiatric diseases; Quantitative susceptibility mapping.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures V. Yedavalli, P. DiGiacomo, E. Tong: no disclosures. M. Zeineh received research funding from GE Healthcare.
Figures





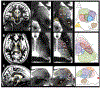
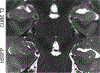
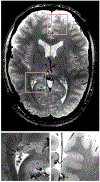

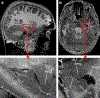
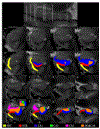


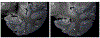
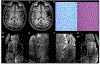
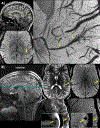

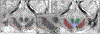





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

