Immunoglobulin deficiency as an indicator of disease severity in patients with COVID-19
- PMID: 33237794
- PMCID: PMC8057306
- DOI: 10.1152/ajplung.00359.2020
Immunoglobulin deficiency as an indicator of disease severity in patients with COVID-19
Abstract
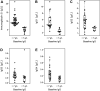
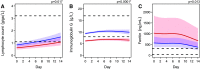
Despite the pandemic status of COVID-19, there is limited information about host risk factors and treatment beyond supportive care. Immunoglobulin G (IgG) could be a potential treatment target. Our aim was to determine the incidence of IgG deficiency and associated risk factors in a cohort of 62 critically ill patients with COVID-19 admitted to two German ICUs (72.6% male, median age: 61 yr). Thirteen (21.0%) of the patients displayed IgG deficiency (IgG < 7 g/L) at baseline (predominant for the IgG1, IgG2, and IgG4 subclasses). Patients who were IgG-deficient had worse measures of clinical disease severity than those with normal IgG levels (shorter duration from disease onset to ICU admission, lower ratio of [Formula: see text] to [Formula: see text], higher Sequential Organ Failure Assessment score, and higher levels of ferritin, neutrophil-to-lymphocyte ratio, and serum creatinine). Patients who were IgG-deficient were also more likely to have sustained lower levels of lymphocyte counts and higher levels of ferritin throughout the hospital stay. Furthermore, patients who were IgG-deficient compared with those with normal IgG levels displayed higher rates of acute kidney injury (76.9% vs. 26.5%; P = 0.001) and death (46.2% vs. 14.3%; P = 0.012), longer ICU [28 (6-48) vs. 12 (3-18) days; P = 0.012] and hospital length of stay [30 (22-50) vs. 18 (9-24) days; P = 0.004]. Univariable logistic regression showed increasing odds of 90-day overall mortality associated with IgG-deficiency (odds ratio 5.14, 95% confidence interval 1.3-19.9; P = 0.018). IgG deficiency might be common in patients with COVID-19 who are critically ill, and warrants investigation as both a marker of disease severity as well as a potential therapeutic target.
Keywords: cytokine release syndrome; immunodysregulation; respiratory failure; severe acute respiratory syndrome coronavirus 2.
Conflict of interest statement
W. Seeger discloses the receipt of personal fees for consultations from Bayer Pharma AG, Liquidia Technologies Inc., and United Therapeutics Corporation, but these consultations were not related to the submitted work. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures


References
-
- World Health Organization. Coronavirus disease (COVID-2019) situation reports (Online). https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... [2020 October 1].
-
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062, 2020. doi:10.1016/S0140-6736(20)30566-3. - DOI - PMC - PubMed
-
- Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130: 2620–2629, 2020. doi:10.1172/JCI137244. - DOI - PMC - PubMed
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi:10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

