Multimodality cardiac imaging in the 21st century: evolution, advances and future opportunities for innovation
- PMID: 33237824
- PMCID: PMC7774683
- DOI: 10.1259/bjr.20200780
Multimodality cardiac imaging in the 21st century: evolution, advances and future opportunities for innovation
Abstract
Cardiovascular imaging has significantly evolved since the turn of the century. Progress in the last two decades has been marked by advances in every modality used to image the heart, including echocardiography, cardiac magnetic resonance, cardiac CT and nuclear cardiology. There has also been a dramatic increase in hybrid and fusion modalities that leverage the unique capabilities of two imaging techniques simultaneously, as well as the incorporation of artificial intelligence and machine learning into the clinical workflow. These advances in non-invasive cardiac imaging have guided patient management and improved clinical outcomes. The technological developments of the past 20 years have also given rise to new imaging subspecialities and increased the demand for dedicated cardiac imagers who are cross-trained in multiple modalities. This state-of-the-art review summarizes the evolution of multimodality cardiac imaging in the 21st century and highlights opportunities for future innovation.
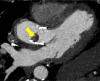
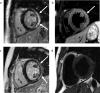
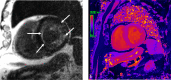
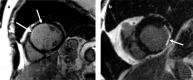
Figures
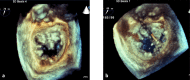
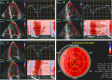
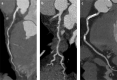
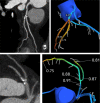


References
-
- Thavendiranathan P, Liu S, Verhaert D, Calleja A, Nitinunu A, Van Houten T, et al. . Feasibility, accuracy, and reproducibility of real-time full-volume 3D transthoracic echocardiography to measure LV volumes and systolic function: a fully automated endocardial contouring algorithm in sinus rhythm and atrial fibrillation. JACC Cardiovasc Imaging 2012; 5: 239–51. doi: 10.1016/j.jcmg.2011.12.012 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

