Development of the Advancing the Patient Experience in COPD Registry: A Modified Delphi Study
- PMID: 33238085
- PMCID: PMC8047610
- DOI: 10.15326/jcopdf.2020.0154
Development of the Advancing the Patient Experience in COPD Registry: A Modified Delphi Study
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is commonly managed by family physicians, but little is known about specifics of management and how this may be improved. The Advancing the Patient Experience in COPD (APEX COPD) registry will be the first U.S. primary care, health system-based registry following patients diagnosed with COPD longitudinally, using a standardized set of variables to investigate how patients are managed in real life and assess outcomes of various management strategies.
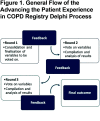
Objective: Gaining expert consensus on a standardized list of variables to capture in the APEX COPD registry.
Methods: A modified, Delphi process was used to reach consensus on which data to collect in the registry from electronic health records (EHRs), patient-reported information (PRI) and patient-reported outcomes (PRO), and by physicians during subsequent office visits. The Delphi panel comprised 14 primary care and specialty COPD experts from the United States and internationally. The process consisted of 3 iterative rounds. Responses were collected electronically.
Results: Of the initial 195 variables considered, consensus was reached to include up to 115 EHR variables, 34 PRI/PRO variables and 5 office-visit variables in the APEX COPD registry. These should include information on symptom burden, diagnosis, COPD exacerbations, lung function, quality of life, comorbidities, smoking status/history, treatment specifics (including side effects), inhaler management, and patient education/self-management.
Conclusion: COPD experts agreed upon the core variables to collect from EHR data and from patients to populate the APEX COPD registry. Data will eventually be integrated, standardized and stored in the APEX COPD database and used for approved COPD-related research.
Keywords: clinically relevant data collection; patient-reported outcomes; primary care; registry; research.
JCOPDF © 2021.
Conflict of interest statement
Chelsea Edwards is an employee of the company Optimum Patient Care, which is a co-founder of the APEX COPD initiative. Alan Kaplan is a member of the advisory board of, or speakers bureau for, Astra Zeneca, Boehringer Ingelheim, Grifols, GlaxoSmithKline, Merck Frosst, Novo Nordisk, Novartis, Paladdin, Pfizer, Purdue, Sanofi, Teva, and Trudel. Barbara Yawn has served on COPD-related advisory boards for GlaxoSmithKline, AstraZeneca, Novartis, and Boehringer Ingelheim, and received COPD-related investigator-initiated research funds from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, and Novartis. Janwillem W. H. Kocks declares grants and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis, and grants from Chiesi, Mundipharma and Teva. Lakmini Bulathsinhala is an employee of the company Optimum Patient Care, which is a co-founder of the APEX COPD initiative. Victoria Carter is an employee of the company Optimum Patient Care, which is a co-founder of the APEX COPD initiative. Ku-Lang Chang and Chester Fox declare no conflicts of interest. Gokul Gopalan is a former employee of Boehringer Ingelheim, which is a co-founder of the APEX COPD initiative and current employee of Vertex Pharmaceuticals. MeiLan Han reports consulting for Boehringer Ingelheim, GlaxoSmithKline and AstraZeneca, and research support from Novartis and Sunovion. Maja Kruszyk is an employee of the company Optimum Patient Care, which is a co-founder of the APEX COPD initiative. Chantal Le Lievre is an employee of the company Optimum Patient Care, which is a co-founder of the APEX COPD initiative. Cathy Mahle is an employee of the company Boehringer Ingelheim, which is a co-founder of the APEX COPD initiative. Barry Make reports funding from the National Heart, Lung and Blood Institute for the COPD Genetic Epidemiology study; grants and medical advisory boards from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, and Sunovian; personal fees for DSMB from Spiration and Shire/Baxalta; CME personal fees from WebMD, National Jewish Health, American College of Chest Physicians, Projects in Knowledge, Hybrid Communications, SPIRE Learning, Ultimate Medical Academy, Catamount Medical, Eastern Pulmonary Society, Catamount Medical Communications Medscape, Eastern VA Medical Center, Academy Continued Healthcare Learning, and Mt. Sinai Medical Center; royalites from Up-To-Date; medical advisory boards from Novartis, Phillips, Third Pole, Science 24/7, and Vernoa; and grants from Pearl outside the submitted work. Wilson Pace is on the advisory board for Mylan and has stock from Novo Nordisk, Pfizer, Novartis, Johnson and Johnson, Stryker, Amgen, Gilead, and Sanofi. Chris Price is an employee of the company Optimum Patient Care, which is a co-founder of the APEX COPD initiative. Asif Shaikh is an employee of the company Boehringer Ingelheim, which is a co-founder of the APEX COPD initiative. Neil Skolnik is on advisory boards for AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline and has received payment for lectures/speaking engagements from AstraZeneca and Boehringer Ingelheim and research support from Sanofi, AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline. David Price has board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals, Thermofisher; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Novartis, Pfizer, Teva Pharmaceuticals, Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma, Novartis; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, Thermofisher; funding for patient enrollment or completion of research from Novartis; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); and is a peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme, and Health Technology Assessment.
References
-
- Mannino DM,Gagnon RC,Petty TL,Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160(11):1683-1689. doi: https://doi.org/10.1001/archinte.160.11.1683 - PubMed
-
- Ma J,Ward EM,Siegel RL,Jemal A. Temporal Trends in Mortality in the United States, 1969-2013. JAMA. 2015;314(16):1731-1739. doi: https://doi.org/10.1001/jama.2015.12319 - PubMed
-
- Lozano R,Naghavi M,Foreman K,et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet Lond Engl. 2012;380(9859):2095-2128. doi: https://doi.org/10.1016/S0140-6736(12)61728-0 - PMC - PubMed
-
- Murray CJL,Atkinson C,Bhalla K,et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591-608. doi: https://doi.org/10.1001/jama.2013.13805 - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous