Design and Applications of Water-Soluble Coordination Cages
- PMID: 33238092
- PMCID: PMC7760102
- DOI: 10.1021/acs.chemrev.0c00672
Design and Applications of Water-Soluble Coordination Cages
Abstract
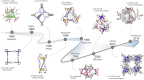
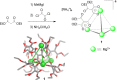
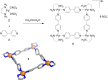
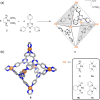
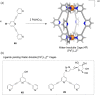
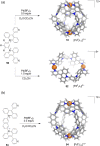
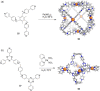
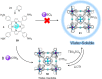
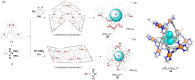
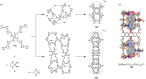
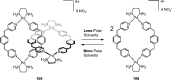
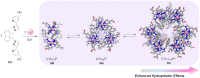
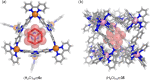
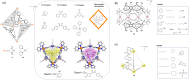
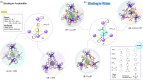
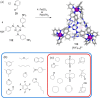
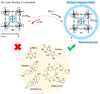
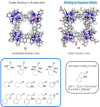
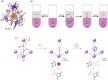
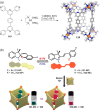
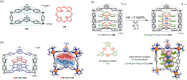
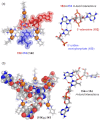
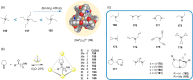
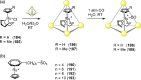
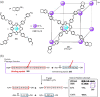
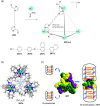
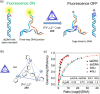
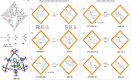
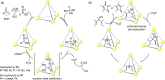
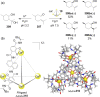
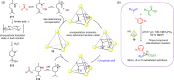
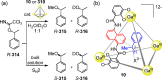
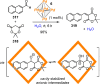
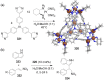
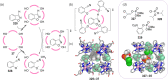
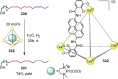
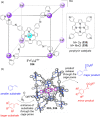
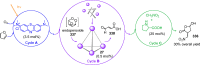
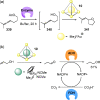
Compartmentalization of the aqueous space within a cell is necessary for life. In similar fashion to the nanometer-scale compartments in living systems, synthetic water-soluble coordination cages (WSCCs) can isolate guest molecules and host chemical transformations. Such cages thus show promise in biological, medical, environmental, and industrial domains. This review highlights examples of three-dimensional synthetic WSCCs, offering perspectives so as to enhance their design and applications. Strategies are presented that address key challenges for the preparation of coordination cages that are soluble and stable in water. The peculiarities of guest binding in aqueous media are examined, highlighting amplified binding in water, changing guest properties, and the recognition of specific molecular targets. The properties of WSCC hosts associated with biomedical applications, and their use as vessels to carry out chemical reactions in water, are also presented. These examples sketch a blueprint for the preparation of new metal-organic containers for use in aqueous solution, as well as guidelines for the engineering of new applications in water.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
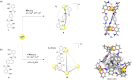
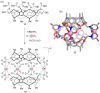
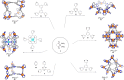
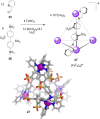



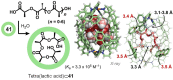
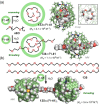
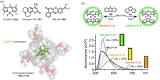
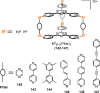


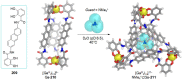
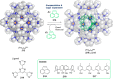
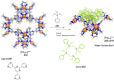
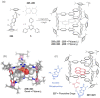



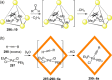
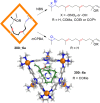
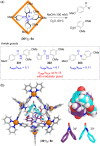
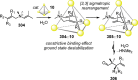
References
Publication types
LinkOut - more resources
Full Text Sources

