Shigella flexneri Disruption of Cellular Tension Promotes Intercellular Spread
- PMID: 33238111
- PMCID: PMC7792532
- DOI: 10.1016/j.celrep.2020.108409
Shigella flexneri Disruption of Cellular Tension Promotes Intercellular Spread
Abstract
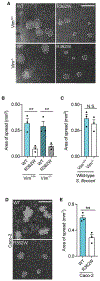
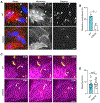
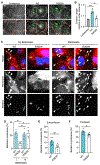
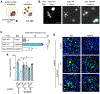
During infection, some bacterial pathogens invade the eukaryotic cytosol and spread between cells of an epithelial monolayer. Intercellular spread occurs when these pathogens push against the plasma membrane, forming protrusions that are engulfed by adjacent cells. Here, we show that IpaC, a Shigella flexneri type 3 secretion system protein, binds the host cell-adhesion protein β-catenin and facilitates efficient protrusion formation. S. flexneri producing a point mutant of IpaC that cannot interact with β-catenin is defective in protrusion formation and spread. Spread is restored by chemical reduction of intercellular tension or genetic depletion of β-catenin, and the magnitude of the protrusion defect correlates with membrane tension, indicating that IpaC reduces membrane tension, which facilitates protrusion formation. IpaC stabilizes adherens junctions and does not alter β-catenin localization at the membrane. Thus, Shigella, like other bacterial pathogens, reduces intercellular tension to efficiently spread between cells.
Keywords: IpaC; Shigella flexneri; cell-to-cell spread; intercellular spread; intracellular pathogens; plasma membrane; type 3 secretion system; β-catenin.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
References
-
- Allaoui A, Mounier J, Prévost MC, Sansonetti PJ, and Parsot C (1992). icsB: a Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol. Microbiol 6, 1605–1616. - PubMed
-
- Allaoui A, Sansonetti PJ, Ménard R, Barzu S, Mounier J, Phalipon A, and Parsot C (1995). MxiG, a membrane protein required for secretion of Shigella spp. Ipa invasins: involvement in entry into epithelial cells and in intercellular dissemination. Mol. Microbiol 17, 461–470. - PubMed
-
- Campbell-Valois FX, Schnupf P, Nigro G, Sachse M, Sansonetti PJ, and Parsot C (2014). A fluorescent reporter reveals on/off regulation of the Shigella type III secretion apparatus during entry and cell-to-cell spread. Cell Host Microbe 15, 177–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

