CRISPR-Cas9: A Preclinical and Clinical Perspective for the Treatment of Human Diseases
- PMID: 33238136
- PMCID: PMC7854284
- DOI: 10.1016/j.ymthe.2020.09.028
CRISPR-Cas9: A Preclinical and Clinical Perspective for the Treatment of Human Diseases
Abstract
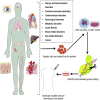
At present, the idea of genome modification has revolutionized the modern therapeutic research era. Genome modification studies have traveled a long way from gene modifications in primary cells to genetic modifications in animals. The targeted genetic modification may result in the modulation (i.e., either upregulation or downregulation) of the predefined gene expression. Clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated nuclease 9 (Cas9) is a promising genome-editing tool that has therapeutic potential against incurable genetic disorders by modifying their DNA sequences. In comparison with other genome-editing techniques, CRISPR-Cas9 is simple, efficient, and very specific. This enabled CRISPR-Cas9 genome-editing technology to enter into clinical trials against cancer. Besides therapeutic potential, the CRISPR-Cas9 tool can also be applied to generate genetically inhibited animal models for drug discovery and development. This comprehensive review paper discusses the origin of CRISPR-Cas9 systems and their therapeutic potential against various genetic disorders, including cancer, allergy, immunological disorders, Duchenne muscular dystrophy, cardiovascular disorders, neurological disorders, liver-related disorders, cystic fibrosis, blood-related disorders, eye-related disorders, and viral infection. Finally, we discuss the different challenges, safety concerns, and strategies that can be applied to overcome the obstacles during CRISPR-Cas9-mediated therapeutic approaches.
Keywords: CRISPR-Cas9; drug development; genome editing; human diseases; therapeutics.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Chakraborty C., Teoh S.L., Das S. The smart programmable CRISPR technology: a next generation genome editing tool for investigators. Curr. Drug Targets. 2017;18:1653–1663. - PubMed
-
- Peng R., Lin G., Li J. Potential pitfalls of CRISPR/Cas9-mediated genome editing. FEBS J. 2016;283:1218–1231. - PubMed
-
- Bortesi L., Fischer R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 2015;33:41–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical