Adenosine A2A and A3 Receptors as Targets for the Treatment of Hypertensive-Diabetic Nephropathy
- PMID: 33238361
- PMCID: PMC7700226
- DOI: 10.3390/biomedicines8110529
Adenosine A2A and A3 Receptors as Targets for the Treatment of Hypertensive-Diabetic Nephropathy
Abstract
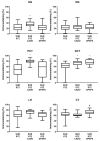
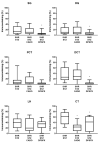
Diabetic nephropathy (DN) and hypertension are prime causes for end-stage renal disease (ESRD) that often coexist in patients, but are seldom studied in combination. Kidney adenosine levels are markedly increased in diabetes, and the expression and function of renal adenosine receptors are altered in experimental diabetes. The aim of this work is to explore the impact of endogenous and exogenous adenosine on the expression/distribution profile of its receptors along the nephron of hypertensive rats with experimentally-induced diabetes. Using spontaneously hypertensive (SHR) rats rendered diabetic with streptozotocin (STZ), we show that treatment of SHR-STZ rats with an agonist of adenosine receptors increases A2A immunoreactivity in superficial glomeruli (SG), proximal tubule (PCT), and distal tubule (DCT). Differently, treatment of SHR-STZ rats with a xanthinic antagonist of adenosine receptors decreases adenosine A3 immunoreactivity in SG, PCT, DCT, and collecting duct. There is no difference in the immunoreactivity against the adenosine A1 and A2B receptors between the experimental groups. The agonist of adenosine receptors ameliorates renal fibrosis, probably via A2A receptors, while the antagonist exacerbates it, most likely due to tonic activation of A3 receptors. The reduction in adenosine A3 immunoreactivity might be due to receptor downregulation in response to prolonged activation. Altogether, these results suggest an opposite regulation exerted by endogenous and exogenous adenosine upon the expression of its A2A and A3 receptors along the nephron of hypertensive diabetic rats, which has a functional impact and should be taken into account when considering novel therapeutic targets for hypertensive-diabetic nephropathy.
Keywords: adenosine receptors; diabetes; diabetic complications; diabetic nephropathy; hypertension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Oyarzun C., Garrido W., Alarcon S., Yanez A., Sobrevia L., Quezada C., San Martin R. Adenosine contribution to normal renal physiology and chronic kidney disease. Mol. Aspects Med. 2017;55:75–89. - PubMed
-
- Olivera A., Lamas S., Rodriguez-Puyol D., Lopez-Novoa J.M. Adenosine induces mesangial cell contraction by an a1-type receptor. Kidney Int. 1989;35:1300–1305. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

