Oxoglutarate Carrier Inhibition Reduced Melanoma Growth and Invasion by Reducing ATP Production
- PMID: 33238375
- PMCID: PMC7700517
- DOI: 10.3390/pharmaceutics12111128
Oxoglutarate Carrier Inhibition Reduced Melanoma Growth and Invasion by Reducing ATP Production
Abstract
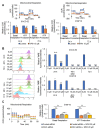
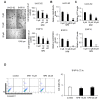
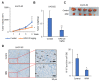
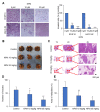
Recent findings indicate that (a) mitochondria in proliferating cancer cells are functional, (b) cancer cells use more oxygen than normal cells for oxidative phosphorylation, and (c) cancer cells critically rely on cytosolic NADH transported into mitochondria via the malate-aspartate shuttle (MAS) for ATP production. In a spontaneous lung cancer model, tumor growth was reduced by 50% in heterozygous oxoglutarate carrier (OGC) knock-out mice compared with wild-type counterparts. To determine the mechanism through which OGC promotes tumor growth, the effects of the OGC inhibitor N-phenylmaleimide (NPM) on mitochondrial activity, oxygen consumption, and ATP production were evaluated in melanoma cell lines. NPM suppressed oxygen consumption and decreased ATP production in melanoma cells in a dose-dependent manner. NPM also reduced the proliferation of melanoma cells. To test the effects of NPM on tumor growth and metastasis in vivo, NPM was administered in a human melanoma xenograft model. NPM reduced tumor growth by approximately 50% and reduced melanoma invasion by 70% at a dose of 20 mg/kg. Therefore, blocking OGC activity may be a useful approach for cancer therapy.
Keywords: ATP production; cancer metabolism; malate-aspartate shuttle; oxoglutarate carrier.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Greenhouse W.V., Lehninger A.L. Occurrence of the malate-aspartate shuttle in various tumor types. Cancer Res. 1976;36:1392–1396. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

