Structural Similarities between Some Common Fluorophores Used in Biology, Marketed Drugs, Endogenous Metabolites, and Natural Products
- PMID: 33238416
- PMCID: PMC7700180
- DOI: 10.3390/md18110582
Structural Similarities between Some Common Fluorophores Used in Biology, Marketed Drugs, Endogenous Metabolites, and Natural Products
Abstract
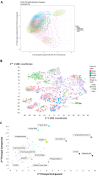
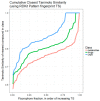
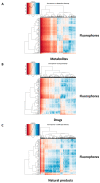
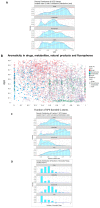
It is known that at least some fluorophores can act as 'surrogate' substrates for solute carriers (SLCs) involved in pharmaceutical drug uptake, and this promiscuity is taken to reflect at least a certain structural similarity. As part of a comprehensive study seeking the 'natural' substrates of 'orphan' transporters that also serve to take up pharmaceutical drugs into cells, we have noted that many drugs bear structural similarities to natural products. A cursory inspection of common fluorophores indicates that they too are surprisingly 'drug-like', and they also enter at least some cells. Some are also known to be substrates of efflux transporters. Consequently, we sought to assess the structural similarity of common fluorophores to marketed drugs, endogenous mammalian metabolites, and natural products. We used a set of some 150 fluorophores along with standard fingerprinting methods and the Tanimoto similarity metric. Results: The great majority of fluorophores tested exhibited significant similarity (Tanimoto similarity > 0.75) to at least one drug, as judged via descriptor properties (especially their aromaticity, for identifiable reasons that we explain), by molecular fingerprints, by visual inspection, and via the "quantitative estimate of drug likeness" technique. It is concluded that this set of fluorophores does overlap with a significant part of both the drug space and natural products space. Consequently, fluorophores do indeed offer a much wider opportunity than had possibly been realised to be used as surrogate uptake molecules in the competitive or trans-stimulation assay of membrane transporter activities.
Keywords: Tanimoto distance; cheminformatics; drugs; fingerprinting; fluorophores; natural products; rdkit; similarity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Understanding the foundations of the structural similarities between marketed drugs and endogenous human metabolites.Front Pharmacol. 2015 May 13;6:105. doi: 10.3389/fphar.2015.00105. eCollection 2015. Front Pharmacol. 2015. PMID: 26029108 Free PMC article.
-
'Metabolite-likeness' as a criterion in the design and selection of pharmaceutical drug libraries.Drug Discov Today. 2009 Jan;14(1-2):31-40. doi: 10.1016/j.drudis.2008.10.011. Epub 2008 Dec 26. Drug Discov Today. 2009. PMID: 19049901 Review.
-
MetMaxStruct: A Tversky-Similarity-Based Strategy for Analysing the (Sub)Structural Similarities of Drugs and Endogenous Metabolites.Front Pharmacol. 2016 Aug 22;7:266. doi: 10.3389/fphar.2016.00266. eCollection 2016. Front Pharmacol. 2016. PMID: 27597830 Free PMC article.
-
The Transporter-Mediated Cellular Uptake and Efflux of Pharmaceutical Drugs and Biotechnology Products: How and Why Phospholipid Bilayer Transport Is Negligible in Real Biomembranes.Molecules. 2021 Sep 16;26(18):5629. doi: 10.3390/molecules26185629. Molecules. 2021. PMID: 34577099 Free PMC article. Review.
-
Design of Novel Drug-like Molecules Using Informatics Rich Secondary Metabolites Analysis of Indian Medicinal and Aromatic Plants.Comb Chem High Throughput Screen. 2020;23(10):1113-1131. doi: 10.2174/1386207323666200606211342. Comb Chem High Throughput Screen. 2020. PMID: 32504496
Cited by
-
Analysis of a Library of Escherichia coli Transporter Knockout Strains to Identify Transport Pathways of Antibiotics.Antibiotics (Basel). 2022 Aug 19;11(8):1129. doi: 10.3390/antibiotics11081129. Antibiotics (Basel). 2022. PMID: 36009997 Free PMC article.
-
FMO-guided design of darunavir analogs as HIV-1 protease inhibitors.Sci Rep. 2024 Feb 13;14(1):3639. doi: 10.1038/s41598-024-53940-1. Sci Rep. 2024. PMID: 38351065 Free PMC article.
-
An Overview of Cell-Based Assay Platforms for the Solute Carrier Family of Transporters.Front Pharmacol. 2021 Aug 10;12:722889. doi: 10.3389/fphar.2021.722889. eCollection 2021. Front Pharmacol. 2021. PMID: 34447313 Free PMC article. Review.
-
Characterization of the Chaetae Autofluorescence in the Earthworm Eisenia foetida and their Role in Locomotion.J Fluoresc. 2025 Jun 21. doi: 10.1007/s10895-025-04374-z. Online ahead of print. J Fluoresc. 2025. PMID: 40542987
-
Discovering Potential Compounds for Venous Disease Treatment through Virtual Screening and Network Pharmacology Approach.Molecules. 2023 Dec 5;28(24):7937. doi: 10.3390/molecules28247937. Molecules. 2023. PMID: 38138427 Free PMC article.
References
-
- Chalfie M., Kain S. Green Fluorescent Protein: Properties, Applications, and Protocols. Wiley-Liss; New York, NY, USA: 1998.
-
- Hemmilä I.A. Applications of Fluorescence in Immunoassays. Wiley; New York, NY, USA: 1991.
-
- Waggoner A.S. Fluorescent probes for cytometry. In: Melamed M.R., Lindmo T., Mendelsohn M.L., editors. Flow Cytometry and Sorting. 2nd ed. Wiley-Liss Inc.; New York, NY, USA: 1990. pp. 209–225.
-
- Kyrychenko A. Using fluorescence for studies of biological membranes: A review. Methods Appl. Fluoresc. 2015;3:042003. - PubMed
-
- Lagorio M.G., Cordon G.B., Iriel A. Reviewing the relevance of fluorescence in biological systems. Photochem. Photobiol. Sci. 2015;14:1538–1559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

